Figure 5.
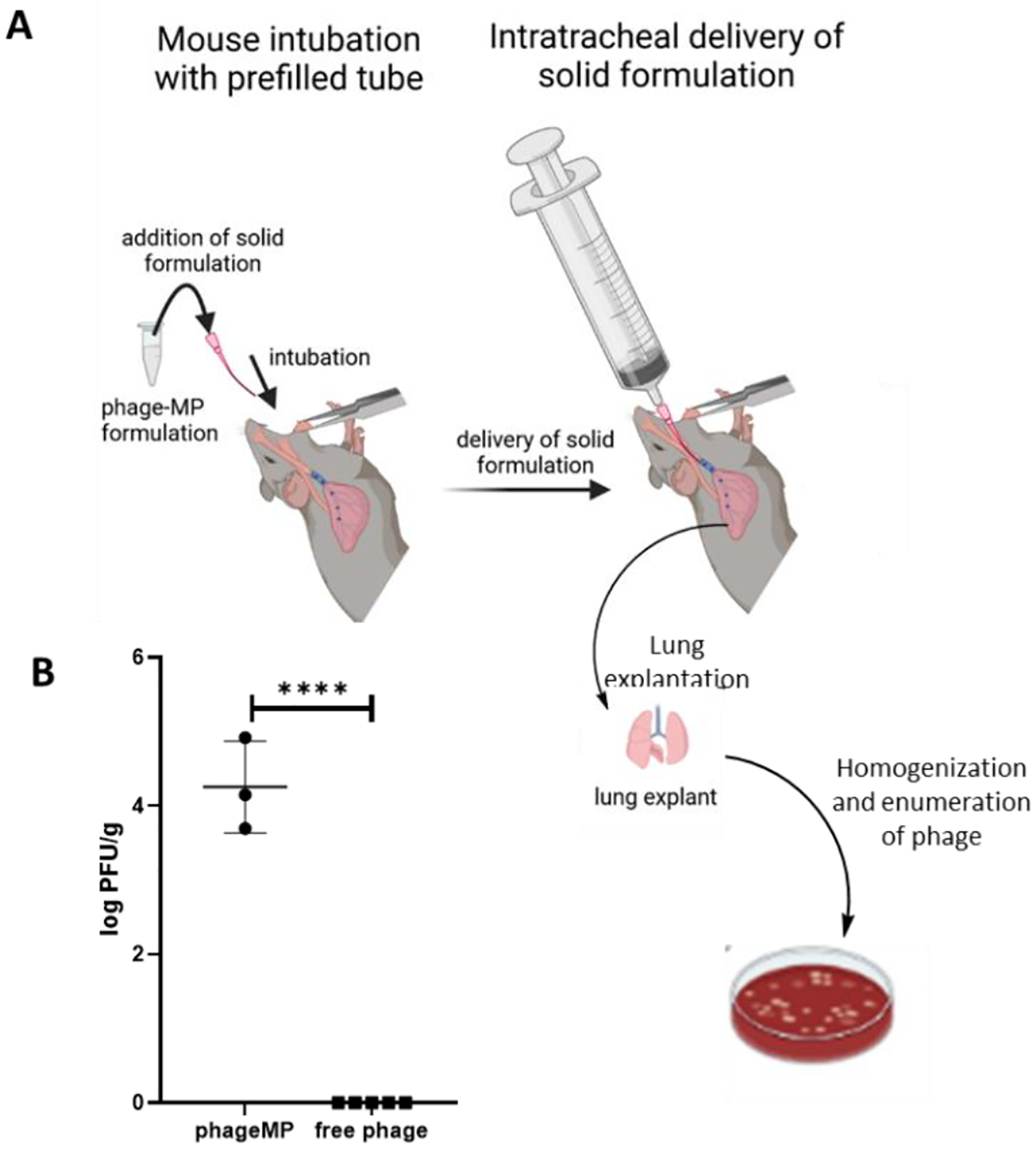
Delivery of phageK-MPs as a dry powder formulation in presence or absence of MPs to the lungs of mice. A, Schematic of endotracheal delivery of the dry powder formulation (Created with BioRender.com). B, Phage recovered in the lungs post-delivery of phage-MPs or free phage. Dry powder formulation was prepared by mixing lyophilized phage K-MPs with respiratory grade lactose (1:9 by weight). Free phage formulation consisted of phage K and lactose (no MPs). A dose of 7.5 × 106 PFU/mouse was delivered to each mouse. ****P < 0.0001 via unpaired two-tailed t test, N = 3–5.
