Abstract
Background:
The aim of this study was to develop a prediction model for 10-year overall survival (OS) after resection of colorectal liver metastasis (CRLM) based on patient, tumor, and treatment characteristics.
Methods:
Consecutive patients after complete resection of CRLM were included from two centers (1992–2019). A prediction model providing 10-year OS probabilities was developed using Cox regression analysis including KRAS, BRAF, and histopathological growth patterns. Discrimination and calibration were assessed using cross-validation. A web-based calculator was built to predict individual 10-year OS probabilities.
Results:
A total of 4112 patients were included. The estimated 10-year OS was 30% (95% CI 29–32). Fifteen patient, tumor, and treatment characteristics were independent prognostic factors for 10-year OS; age, gender, location and nodal status of the primary tumor, disease-free interval, number and diameter of CRLM, preoperative CEA, resection margin, extrahepatic disease, KRAS and BRAF mutation status, histopathological growth patterns, perioperative systemic chemotherapy, and hepatic arterial infusion pump chemotherapy. The discrimination at 10-years was 0.73 for both centers. A simplified risk score identified four risk groups with a 10-year OS of 57%, 38%, 24%, and 12%.
Conclusions:
Ten-year OS after resection of CRLM is best predicted with a model including 15 patient, tumor, and treatment characteristics. The web-based calculator can be used to inform patients. This model serves as a benchmark to determine the prognostic value of novel biomarkers.
Keywords: Colorectal liver metastases, prognostic factors, biomarkers, prediction
Introduction
Survival beyond 10-years after resection of colorectal liver metastases (CRLM) reflects cure in most (98%) patients.(1, 2) While most recurrences occur within the first two years after resection, patients continue to be at risk for recurrence in subsequent years.(1) Five-year overall survival (OS) is typically reported in randomized controlled trials (RCTs), but does not reflect cure. Most studies reported a 5-year survival of roughly 50% and a 10-year survival of 25% after resection of CRLM. Ten-year estimates, however, vary across a wide range due to small sample size and limited follow-up of most studies.(2–7)
Known unfavorable prognostic factors associated with 10-year survival include node-positive primary colorectal cancer (CRC), right-sided CRC, synchronous presentation, multiple metastases, CRLM large in size, high serum carcinoembryonic antigen (CEA) levels, presence of extrahepatic disease, and positive resection margins. However, no single unfavorable factor precludes 10-year OS.(1, 2) Biomarkers such as KRAS, BRAF, and histological growth pattern (HGP) further improve prognostication after resection of CRLM. These biomarkers have been included in models to predict 5-year OS, but sample size and length of follow-up were insufficient for 10-year OS.(8–10)
Several RCTs have investigated perioperative systemic chemotherapy and hepatic arterial infusion pump (HAIP) chemotherapy.(11–16) Although long-term survival outcomes of these treatments have been published (17, 18), these treatment factors have yet to be incorporated in prediction models.
The aim of this study was to develop a prediction model for 10-years OS for individual patients with resected CRLM based on patient, tumor, and treatment characteristics.
Materials and Methods
This study was performed according to the TRIPOD guidelines.(19)
Patients
Consecutive patients who underwent resection and/or ablation of CRLM between January 1992 and January 2019 from Memorial Sloan Kettering Cancer Center (MSKCC; New York, USA) and between January 2000 and January 2019 from Erasmus MC Cancer Institute (Rotterdam, the Netherlands) were included.
Perioperative management
Preoperative systemic chemotherapy was administered in patients with borderline resectable or unresectable CRLM for downstaging in both centers. Neoadjuvant systemic chemotherapy (i.e., for resectable CRLM) and adjuvant systemic and/or HAIP chemotherapy were administered at MSKCC at the discretion of the treating physicians.(20–22)
Definitions
Histopathological Growth Patterns (HGPs) were assessed on hematoxylin and eosin slides of resection specimens.(23) Two clinically relevant phenotypes are recognized; a desmoplastic and a non-desmoplastic type. CRLM displaying any replacement or pushing HGP were considered non-desmoplastic.(9) Definitions of all prognostic factors has been published previously.(24)
Statistical analysis
The primary endpoint in the analyses was OS, which was calculated from the time of resection to the time of death or last follow-up. Median follow-up was calculated using the reversed Kaplan-Meier method. Continuous factors were compared using the Mann-Whitney U test and categorical factors using the Chi-square test. Missing data were multiply imputed by chained equations to avoid loss of information due to case-wise deletion.(25) Multivariable Cox proportional hazards regression analyses were performed including predictors that were known to be associated with OS.(26) Three-knot restricted cubic splines were used to assess linearity of continuous factors.(27)
The model discrimination was evaluated by the time-dependent area under the receiver operating characteristic (ROC) curve (AUC). The AUC was based on weighting by the inverse probability of censoring at 10-years.(28) Discrimination was evaluated with a leave-one-study-out cross-validation. That is, in each validation step, one center is used to develop the model while the other is used as a validation set to provide the performance of the models in the two centers, although both centers were used to estimate the absolute risk.(29) External validity was assessed according to the AUCs of both centers. Calibration was assessed visually by plotting the predicted probability against the actual observed frequency of predicted outcomes at 10 years.(30) The discriminative power of the model was compared with the clinical risk score (CRS) and the GAME score using the likelihood ratio test.(8, 31)
A separate multivariable model with dichotomized factors was used to develop a simplified risk score. Backward selection with stepwise elimination of factors with a p-value >0.20 was performed. Points for the score were determined by multiplying each regression coefficient by 5 rounded to integers.(32) Next, four risk groups were proposed based on observed 10-year OS probabilities.
A p-value of <0.05 was considered as statistically significant. Analyses were performed in Rstudio (version 1.0.153, Boston, MA). The protocol of this study was approved by the Institutional Review Board of MSKCC (IRB number 16-533) and Erasmus MC (MEC-2020-0294).
Results
A total of 4539 patients underwent curative-intent surgery for CRLM at the two centers during the study period. Reasons for exclusion were incomplete liver resection (n = 251, 6%), residual extrahepatic disease (n = 124, 3%), and no colorectal resection (n = 52, 1%). The final group included 4112 patients; 3064 patients (75%) from MSKCC (period 1992–2019) and 1048 patients (25%) from Erasmus MC (period 2000–2019).
Patient characteristics
The median age was 61 years (Interquartile range (IQR) 52–69 years, Table 1). The majority of patients (n = 3366, 82%) had a resection since 2000. Extrahepatic disease was resected or ablated before or at the time of resection of CRLM in 468 patients (11%). KRAS mutational status was available in 1567 patients (38%) and mutated in 639 (41%). BRAF mutational status was available in 1358 patients (33%) and mutated in 55 patients (4%). HGP were assessed in 3136 patients (76%), and a desmoplastic HGP was found in 470 patients (22%). Perioperative systemic chemotherapy was administered in 3042 patients (74%); additional HAIP chemotherapy was administered in 1061 patients (26%). During follow-up 2372 patients died. The median follow-up for survivors was 99 months (IQR 53–160 months).
Table 1.
Patient characteristics and 10-year OS probabilities
| Erasmus MC (%) |
MSKCC (%) |
Total (%) |
Median OS | 10-year OS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Months | 95% CI | P-value | % | 95% CI | |||||
| Total number of patients | 1048 (25) | 3064 (75) | 4112 | ||||||
| PATIENT CHARACTERISTICS | |||||||||
| Age | Median (IQR) | 64 (58–71) | 60 (50–68) | 61 (52–69) | <0.001 | ||||
| =<60 years | 349 (33) | 1594 (52) | 1943 (47) | 65 | 60–69 | 35 | 33–38 | ||
| >60 years | 699 (67) | 1470 (48) | 2169 (53) | 56 | 53–59 | 26 | 24–29 | ||
| Gender | 0.19 | ||||||||
| Female | 367 (35) | 1323 (43) | 1690 (41) | 58 | 54–63 | 33 | 29–35 | ||
| Male | 681 (65) | 1741 (57) | 2422 (59) | 60 | 57–65 | 29 | 27–31 | ||
| Year of surgery | <0.001 | ||||||||
| <2000 | 0 | 746 (24) | 746 (18) | 47 | 42–51 | 25 | 22–29 | ||
| ≥2000 | 1048 (100) | 2318 (76) | 3366 (82) | 63 | 60–67 | 32 | 30–34 | ||
| DISEASE CHARACTERISTICS | |||||||||
| Location CRC | <0.001 | ||||||||
| Right-sided | 192 (19) | 836 (28) | 1028 (26) | 49 | 47–54 | 27 | 24–31 | ||
| Left-sided | 454 (44) | 1402 (47) | 1856 (47) | 65 | 61–70 | 33 | 30–35 | ||
| Rectum | 379 (37) | 725 (25) | 1104 (28) | 58 | 55–64 | 29 | 26–33 | ||
| Missing | 23 | 101 | 124 | ||||||
| pT-stage | 0.002 | ||||||||
| 0 * | 26 (3) | 13 (1) | 39 (1) | 94 | 63-NR | 37 | 19–69 | ||
| 1 | 14 (1) | 85 (3) | 99 (3) | 105 | 59–155 | 47 | 36–61 | ||
| 2 | 146 (14) | 287 (10) | 433 (11) | 66 | 58–74 | 35 | 31–41 | ||
| 3 | 731 (71) | 1984 (72) | 2715 (72) | 58 | 56–63 | 30 | 28–32 | ||
| 4 | 113 (11) | 398 (14) | 511 (13) | 50 | 42–58 | 26 | 21–32 | ||
| Missing | 18 | 297 | 315 | ||||||
| pT-stage | 0.003 | ||||||||
| T 0–2 | 186 (18) | 385 (14) | 571 (15) | 67 | 63–82 | 37 | 33–42 | ||
| T 3–4 | 844 (82) | 2382 (86) | 3226 (85) | 57 | 55–61 | 30 | 28–32 | ||
| Missing | 18 | 297 | 315 | ||||||
| Nodal status CRC | <0.001 | ||||||||
| N0 | 421 (41) | 1126 (37) | 1547 (39) | 75 | 71–82 | 39 | 36–42 | ||
| N1 | 392 (38) | 1168 (39) | 1560 (38) | 55 | 52–59 | 26 | 24–29 | ||
| N2 | 211 (21) | 736 (24) | 947 (23) | 47 | 43–50 | 23 | 20–26 | ||
| Missing | 22 | 43 | 65 | ||||||
| Nodal status CRC | <0.001 | ||||||||
| N0 | 420 (41) | 1127 (37) | 1547 (38) | 75 | 71–82 | 39 | 36–42 | ||
| N+ | 609 (59) | 1906 (63) | 2515 (62) | 52 | 50–55 | 25 | 23–27 | ||
| Missing | 19 | 31 | 50 | ||||||
| Extrahepatic disease ** | <0.001 | ||||||||
| No | 940 (90) | 2704 (88) | 3644 (89) | 63 | 60–67 | 32 | 31–34 | ||
| Yes | 108 (10) | 360 (12) | 468 (11) | 40 | 37–45 | 14 | 11–19 | ||
| Disease-free interval | 0.24 | ||||||||
| ≤ 12 months | 734 (70) | 2085 (68) | 2819 (69) | 57 | 55–60 | 30 | 28–32 | ||
| > 12 months | 314 (30) | 973 (32) | 1287 (31) | 64 | 59–70 | 31 | 28–34 | ||
| Missing | 0 | 6 | 6 | ||||||
| Number CRLM | <0.001 | ||||||||
| 1 | 452 (44) | 1252 (41) | 1704 (42) | 71 | 66–76 | 35 | 32–38 | ||
| 2 | 211 (20) | 598 (20) | 809 (20) | 60 | 55–68 | 32 | 29–36 | ||
| 3 | 118 (11) | 391 (13) | 509 (13) | 55 | 49–58 | 29 | 24–34 | ||
| 4 | 89 (9) | 233 (8) | 322 (8) | 51 | 46–58 | 28 | 22–34 | ||
| 5–9 | 139 (13) | 451 (15) | 590 (15) | 47 | 42–52 | 23 | 19–28 | ||
| ≥10 | 29 (3) | 115 (4) | 144 (4) | 38 | 34–48 | 14 | 8–26 | ||
| 10 | 24 | 34 | |||||||
| Size largest tumor | <0.001 | ||||||||
| ≤ 5cm | 823 (83) | 2285 (76) | 3108 (78) | 65 | 62–68 | 33 | 31–36 | ||
| > 5cm | 163 (17) | 729 (24) | 892 (22) | 43 | 39–48 | 22 | 19–26 | ||
| Missing | 62 | 50 | 112 | ||||||
| Preoperative CEA | <0.001 | ||||||||
| ≤ 200 μg/L | 899 (92) | 2497 (91) | 3396 (92) | 61 | 58–65 | 32 | 30–34 | ||
| > 200 μg/L | 80 (8) | 234 (9) | 314 (8) | 48 | 41–51 | 19 | 14–25 | ||
| Missing | 69 | 333 | 402 | ||||||
| Resection margin involved | <0.001 | ||||||||
| No | 844 (85) | 2686 (89) | 3530 (88) | 63 | 59–66 | 32 | 30–34 | ||
| Yes | 155 (15) | 335 (11) | 490 (12) | 42 | 37–46 | 17 | 13–21 | ||
| Missing | 49 | 43 | 92 | ||||||
|
KRAS mutational status
|
<0.001 | ||||||||
| Wildtype | 131 (61) | 797 (59) | 928 (59) | 78 | 72–86 | 34 | 30–39 | ||
| Mutated | 85 (39) | 554 (41) | 639 (41) | 53 | 49–58 | 27 | 22–32 | ||
| Missing | 832 | 1713 | 2545 | ||||||
| BRAF mutational status | <0.001 | ||||||||
| Wildtype | 183 (97) | 1120 (96) | 1303 (96) | 72 | 66–79 | 33 | 29–37 | ||
| Mutated | 6 (3) | 49 (4) | 55 (4) | 40 | 35–70 | 22 | 11–46 | ||
| Missing | 859 | 1895 | 2754 | ||||||
| Histopathological growth pattern | <0.001 | ||||||||
| dHGP | 216 (24) | 254 (21) | 470 (22) | 86 | 75–104 | 41 | 36–48 | ||
| non-dHGP | 696 (76) | 970 (79) | 1666 (78) | 57 | 51–59 | 26 | 23–29 | ||
| Missing | 136 | 1840 | 1976 | ||||||
| TREATMENT CHARACTERISTICS | |||||||||
| Perioperative systemic CTx | <0.001 | ||||||||
| No CTx | 546 (53) | 241 (9) | 787 (21) | 53 | 48–60 | 26 | 22–30 | ||
| 5-FU only | 25 (2) | 549 (20) | 574 (15) | 53 | 50–59 | 28 | 24–32 | ||
| OXA- or IRINO | 461 (45) | 2007 (71) | 2468 (64) | 64 | 60–68 | 34 | 32–36 | ||
| Missing | 16 | 267 | 283 | ||||||
| Perioperative HAIP CTx | <0.001 | ||||||||
| No | 1037 (99) | 2014 (66) | 3051 (74) | 55 | 53–58 | 27 | 25–29 | ||
| Yes | 11 (1) | 1050 (34) | 1061 (26) | 73 | 68–83 | 40 | 36–43 | ||
Abbreviations: CEA: carcinoembryonic antigen, CI: confidence interval, CRC: colorectal cancer, CRLM: colorectal liver metastases, CTx: chemotherapy, HAIP: hepatic arterial infusion pump chemotherapy, HGP: histopathological growth pattern. IRINO: irinotecan, IQR: interquartile range, OXA: oxaliplatin, NR: not reached, pT-stage: pathology tumor stage
Rectal cancer with complete response after neoadjuvant chemoradiotherapy.
Resected prior or during CRLM resection
10-year OS for patient, tumor, and treatment characteristics
Estimated median OS for the whole cohort was 59 months (95% CI 57–62 months) with an estimated 5-year OS of 49% (95% CI 48–51) and a 10-year OS of 30% (95% CI 29–32%). Poor prognostic factors associated with a 10-year OS probability below 20% were extrahepatic disease before or at time of CRLM resection (14%, 95% CI 11–19%), 10 or more CRLM (14%, 95% CI 8–26%), a CEA level of more than 200 μg/L (19%, 95% CI 14–25%), and a positive resection margin (17%, 95% CI 13–21%, Table 1). Favorable prognostic factors associated with a 10-year OS rate above 40% were pT1 CRC (47%, 95% CI 36–61%), and desmoplastic HGP (41%, 95% CI 36–48%). Genomic alterations were associated with a 10-year OS of 27% (95% CI 30–39%) for KRAS mutants and 22% (95% CI 11–46%) for BRAF mutants (Table 1).
Perioperative oxaliplatin- or irinotecan-based systemic chemotherapy was associated with a 10-year OS probability of 34% (95% CI 32–36%), compared to 28% (95% CI 24–32%) for 5-FU only systemic chemotherapy, and 26% (95% CI 22–30%) for no perioperative systemic chemotherapy (p < 0.001). Perioperative HAIP chemotherapy was associated with a 10-year OS probability of 40% (95% CI 36–43%) compared to 27% (95% CI 25–29%) without perioperative HAIP chemotherapy (p < 0.001).
Individual probability of 10-year OS
Fifteen independent prognostic factors for 10-year OS were included in the model; age, gender, location CRC, nodal status CRC, disease-free interval, number of CRLM, diameter of largest CRLM, preoperative CEA, resection margin, extrahepatic disease, KRAS mutation status, BRAF mutation status, histopathological growth pattern, perioperative systemic chemotherapy, and perioperative HAIP chemotherapy (Table 2). The 10-year OS probability for individual patients can be estimated using the web-based calculator (https://dhoppener.shinyapps.io/10yos/) or using the equation in the Supplements. With internal-external cross-validation, the AUC was 0.73 (95% CI: 0.68–0.78) for Erasmus MC and 0.73 (95% CI 0.70–0.75) for MSKCC.
Table 2.
Multivariable Cox regression analysis
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| Age (10-year increase) | 1.31 | 1.23–1.40 | <0.001 |
| Gender (male) | 1.15 | 1.06–1.25 | 0.001 |
| Location CRC | |||
| Right-sided | REF | ||
| Left-sided | 0.90 | 0.81–0.99 | 0.04 |
| Rectum | 1.04 | 0.93–1.17 | 0.45 |
| Node-positive CRC | 1.45 | 1.33–1.59 | <0.001 |
| Disease-free interval | 0.996 | 0.993–0.999 | 0.01 |
| pT-stage (pT3-4) | 1.03 | 0.91–1.15 | 0.65 |
| Number CRLM | 1.11 | 1.10–1.14 | <0.001 |
| Diameter CRLM (cm) | 1.09 | 1.07–1.11 | <0.001 |
| Preoperative CEA level | 1.003 | 1.001–1.005 | 0.004 |
| Positive resection margin | 1.40 | 1.24–1.58 | <0.001 |
| Extrahepatic disease | 1.62 | 1.44–1.83 | <0.001 |
| Perioperative systemic CTx | |||
| No CTx | REF | ||
| 5-FU only | 0.83 | 0.73–0.95 | 0.005 |
| Oxaliplatin or irinotecan | 0.84 | 0.74–0.94 | 0.003 |
| Perioperative HAIP CTx | 0.73 | 0.65–0.81 | <0.001 |
| KRAS mutant | 1.59 | 1.46–1.73 | <0.001 |
| BRAF mutant | 1.69 | 1.42–2.01 | <0.001 |
| Non-dHGP | 1.57 | 1.40–1.77 | <0.001 |
Abbreviations: CEA: carcinoembryonic antigen, CI: confidence interval, CRC: colorectal cancer, CRLM: colorectal liver metastases, CTx: chemotherapy, HAIP: hepatic arterial infusion pump chemotherapy, HGP: histopathological growth pattern, pT-stage: pathology tumor stage
Calibration showed slight overestimation of the model developed in Erasmus MC and validated in MSKCC. Calibration was good in the model developed in MSKCC and validated in Erasmus MC (Figure 1). Interactions between center and all candidate predictors were not statistically significant (p > 0.05).
Figure 1. Calibration plots in the Erasmus and MSKCC cohort.
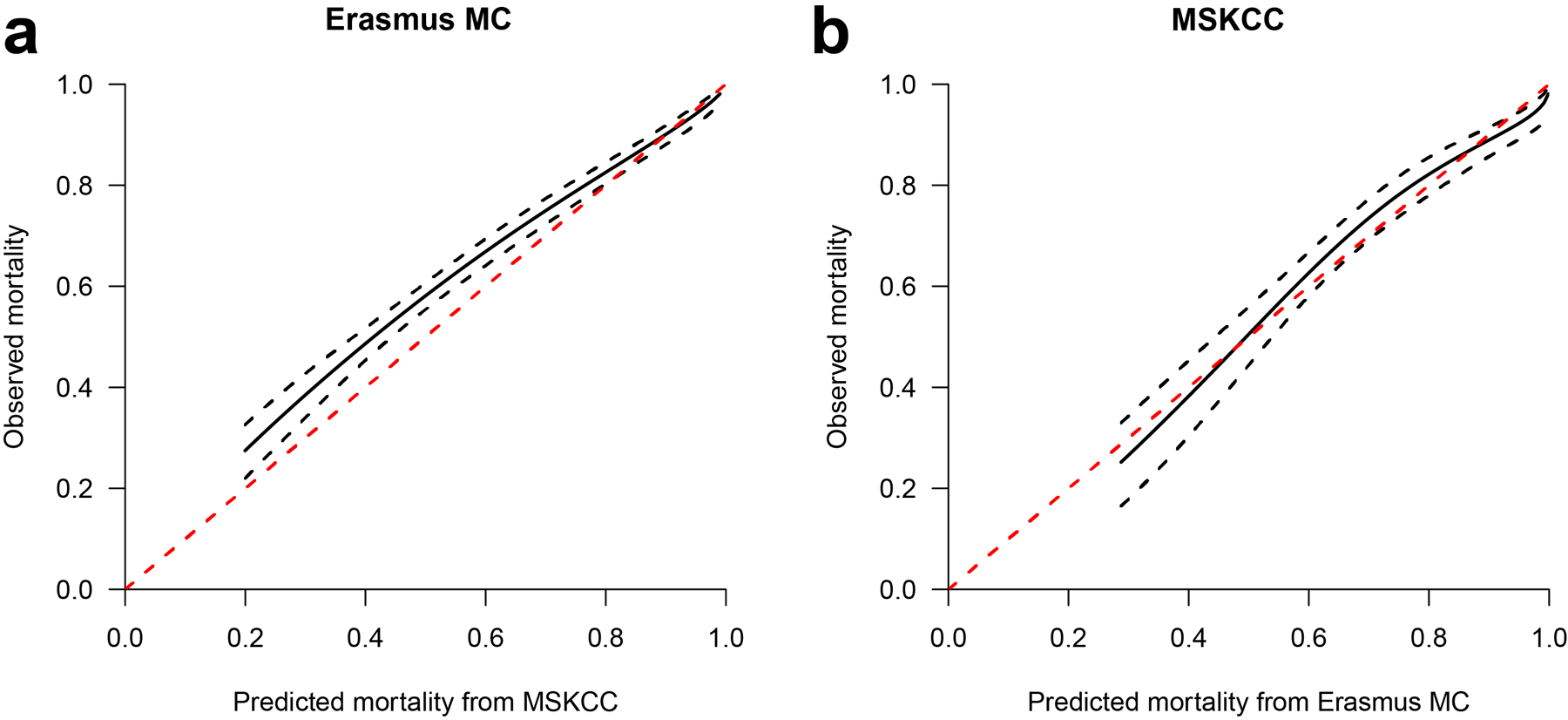
A) Full model developed in Erasmus MC and validated in MSKCC
B) Full model developed in MSKCC and validated in Erasmus MC
The black solid lines represent the predicted and observed mortality. The black dotted lines are the 95% prediction intervals. Perfect calibration would be present if the solid black line would overlap the red dotted line.
This model outperformed the CRS by Fong (AUC 0.62, 95% CI 0.59–0.64, p < 0.001) and the GAME score by Margonis (AUC 0.66, 95% CI 0.64–0.69, p < 0.001).
Simplified risk score
A simplified risk score with 13 dichotomized prognostic factors is presented in Table 3. Disease-free interval and location of the primary CRC dropped out of the dichotomized model. The risk score is calculated by adding points for poor prognostic factors and subtracting points for treatment effects. The cumulative score ranges from −3 to 17. Four groups were identified; favorable (≤ 3 points), intermediate (4–5 points), unfavorable (6–8 points), and very unfavorable (≥9 points) with corresponding 10-year OS (95% CI) probabilities of 57% (51–60%; n = 692), 38% (34–42%, n = 993), 24% (23–29%, n = 1483), and 12% (10–15%, n = 944) respectively (Figure 2).
Table 3.
Simplified risk score for 10-year OS with dichotomized factors
| Prognostic factors | HR | 95% CI | Points | |
|---|---|---|---|---|
| Age > 60 years | 1.31 | 1.20–1.42 | 2 | |
| Gender (male) | 1.15 | 1.06–1.25 | 1 | |
| Node positive CRC | 1.47 | 1.35–1.60 | 2 | |
| More than one CRLM | 1.37 | 1.26–1.50 | 2 | |
| Size CRLM > 5 cm | 1.44 | 1.31–1.58 | 2 | |
| Preoperative CEA > 200 μg/L | 1.13 | 0.99–1.30 | 1 | |
| Positive resection margin | 1.48 | 1.32–1.66 | 2 | |
| Extrahepatic disease | 1.57 | 1.39–1.77 | 3 | |
| KRAS mutant | 1.58 | 1.45–1.72 | 3 | |
| BRAF mutant | 1.74 | 1.46–2.07 | 3 | |
| Non-dHGP | 1.63 | 1.45–1.83 | 3 | |
| Perioperative systemic CTx * | 0.86 | 0.78–0.96 | -1 | |
| Perioperative HAIP CTx | 0.75 | 0.68–0.84 | -2 | |
| Groups | Sample size | 10-year OS (%) | 95% CI | Points |
| Favorable | 711 | 57 | 53–62 | ≤ 3 |
| Intermediate | 952 | 38 | 34–42 | 4 – 5 |
| Unfavorable | 1585 | 24 | 21–26 | 6 – 8 |
| Very unfavorable | 846 | 12 | 10–15 | ≥ 9 |
Abbreviations: CI: confidence interval, CRC: colorectal cancer, CRLM: colorectal liver metastases, CTx: chemotherapy, HAIP: hepatic arterial infusion pump chemotherapy, HR: hazard ratio, non-dHGP: non-desmoplastic histopathological growth pattern
Combining 5-FU-only and oxaliplatin- or irinotecan-based perioperative systemic chemotherapy
Figure 2.

Kaplan-Meier of 4 groups of the simplified risk score
Discussion
We developed a web-based calculator to predict the individual patient’s probability of 10-year OS after resection of CRLM using fifteen prognostic factors. The simplified risk score distinguished four groups with a 10-year OS ranging from 12% to 57%. This is the first clinical prediction model for OS after resection of CRLM that incorporates BRAF and HGP. Moreover, systemic and HAIP chemotherapy were incorporated in addition to patient and tumor characteristics.
Several studies have reported 10-year OS after resection of CRLM.(2–7) In a meta-analysis of eleven studies, together representing 2387 patients, the estimated 10-year OS ranged from 12% to 37%.(3) Two published prognostic models predicted 10-year OS, but considered only a small subset of all known prognostic factors.(2, 4) Moreover, both models were developed using logistic regression analyses, which introduced bias by excluding patients lost to follow-up before 10-years.
Most published models predicted recurrence of disease or short-term OS and had a small sample size below 1000 patients.(8, 31, 33–38) One of the first models was the clinical risk score (CRS) by Fong et al.(30) The score is based on five prognostic factors; DFI, nodal status, number of CRLM, size of CRLM, and preoperative serum CEA level. The strength of the score is its simplicity with one point assigned to each factor. However, the score was developed to predict recurrence, included only 5 factors, and did not consider genomic alterations and treatment. These aspects may explain the poor performance of the CRS score in external validations (range C-index 0.53–0.56).(39–41)
Several studies have demonstrated that KRAS mutation is an important prognostic factor for OS after resection of CRLM.(8, 42–45) In the largest study with 2655 patients in the National Cancer Database, KRAS status was an independent prognostic factor for OS (adjusted HR 1.21 95% CI 1.04–1.39, p = 0.012).(45) Two recent models have included KRAS mutation as a prognostic factor.(8, 37) However, both the GAME model and the model of Goffredo et al. did not account for several known and readily available prognostic patient and tumor factors. The present study confirmed that KRAS is an independent poor prognostic factor for 10-year OS with an adjusted HR of 1.59 (95% CI 1.46–1.73). Moreover, the current model included BRAF mutation as prognostic factor with an adjusted HR of 1.69. The present model clearly outperforms both the CRS and GAME score in discriminative power.
A non-desmoplastic HGP was recently identified as a strong and independent poor prognostic factor.(9) Other studies demonstrated that a non-desmoplastic HGP is associated with positive resection margins and unsalvageable recurrences, both representing aggressive tumor biology.(10, 46, 47) The current study identified HGPs as an independent prognostic factor for 10-year OS with an adjusted HR of 1.6 for non- desmoplastic HGP.
The present model also contains systemic and HAIP chemotherapy. Two RCTs could not demonstrate superior OS of perioperative systemic chemotherapy for patients undergoing resection of CRLM.(17, 48) These studies randomized about 300 patients and included mostly patients with a favorable risk profile (e.g., low number of CRLM). In the present study, perioperative oxaliplatin- or irinotecan-based systemic chemotherapy was an independent favorable factor for 10-year OS with an adjusted HR of 0.84. Perioperative HAIP chemotherapy was associated with a 10-year OS probability of 40%, which was similar to the 10-year OS of 41% in a randomized controlled trial.(18) A 10-year OS of 61% (95% CI 51%−70%) was reported for patients treated with perioperative HAIP chemotherapy after 2003 in clinical trials.(49) Perioperative HAIP chemotherapy was also an independent prognostic factor for 10-year OS with an adjusted HR of 0.73.(15, 18) An ongoing phase III RCT investigates adjuvant HAIP chemotherapy in the current era.(50)
A previous paper investigating 10-year OS after resection of CRLM concluded that none of the poor prognostic risk factors precluded cure.(1, 2) In the present, much larger study, we identified additional independent poor prognostic factors for 10-year OS (i.e., KRAS and BRAF mutation and HGP). Again, none of these factors precluded 10-year OS, although several papers found a very poor prognosis in patients with BRAF mutation.(51, 52) The probability of cure after resection of CRLM was 12% in the worst group of patients who had a combination of many poor prognostic factors (i.e., at least 9 points in the simplified risk score). This justifies curative-intent surgery for selected patients regardless of a combination of poor prognostic factors.
This study has several limitations. Firstly, patient selection and treatment have changed during the long inclusion period required to estimate 10-year OS. However, 82% of patients underwent a resection after 2000 and 15 prognostic factors including two treatment factors largely accounted for changes over time. Secondly, the two centers differed in perioperative treatment. In MSKCC, over 90% of patients received perioperative systemic chemotherapy. Dutch guidelines recommend systemic treatment for patients with (borderline) unresectable disease, but not for resectable disease. Moreover, perioperative HAIP chemotherapy has been performed regularly at MSKCC, whereas at Erasmus MC only during the last year.(20) These differences between centers are in fact also a strength, since the dataset included patients with similar characteristics who did and did not receive perioperative systemic and/or HAIP chemotherapy. This allowed for assessment of treatment effects and improved generalizability (i.e., external validity) of the model, as reflected by good discrimination and calibration at cross-validation. Thirdly, the model may appear applicable only in centers that offer HAIP chemotherapy. However, the model included more than 3000 patients who did not receive perioperative HAIP chemotherapy. The model and simplified score can be applied to patients who did and did not receive perioperative HAIP chemotherapy, as demonstrated by the good cross-validation of the MSK model in the Erasmus MC population. Lastly, genomic alterations in KRAS and BRAF, as well as HGP status were missing for many patients. Missing data was accounted for by multiple imputation, which may have led to bias but is methodologically superior to excluding patients with missing data.(53)
In conclusion, this model with web-based calculator accurately predicts 10-year OS after resection of CRLM based on 15 patient, tumor, genomic, and treatment factors. This model may be used to inform patients and clinicians. Moreover, it serves as a benchmark for evaluation of future prognostic biomarkers.
Supplementary Material
Footnotes
Conflict of Interest and Source of Funding
None were declared
References
- 1.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(29):4575–80. [DOI] [PubMed] [Google Scholar]
- 2.Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163(6):1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN Oncol. 2011;2011:763245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulik U, Plohmann-Meyer M, Gwiasda J, Kolb J, Meyer D, Kaltenborn A, et al. Proposal of Two Prognostic Models for the Prediction of 10-Year Survival after Liver Resection for Colorectal Metastases. HPB Surg. 2018;2018:5618581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucchetti A, Ferrero A, Cescon M, Donadon M, Russolillo N, Ercolani G, et al. Cure model survival analysis after hepatic resection for colorectal liver metastases. Annals of surgical oncology. 2015;22(6):1908–14. [DOI] [PubMed] [Google Scholar]
- 6.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galjart B, van der Stok EP, Rothbarth J, Grunhagen DJ, Verhoef C. Posttreatment Surveillance in Patients with Prolonged Disease-Free Survival After Resection of Colorectal Liver Metastasis. Annals of surgical oncology. 2016;23(12):3999–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margonis GA, Sasaki K, Gholami S, Kim Y, Andreatos N, Rezaee N, et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galjart B, Nierop PMH, van der Stok EP, van den Braak R, Hoppener DJ, Daelemans S, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22(2):355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Höppener DJ, Galjart B, Nierop PMH, Buisman FE, van der Stok EP, Coebergh van den Braak RRJ, et al. Histopathological Growth Patterns and Survival After Resection of Colorectal Liver Metastasis: An External Validation Study. JNCI Cancer Spectr. 2021;5(3):pkab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. The Lancet Oncology. 2014;15(6):601–11. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. The New England journal of medicine. 1999;341(27):2039–48. [DOI] [PubMed] [Google Scholar]
- 14.Ayez N, van der Stok EP, de Wilt H, Radema SA, van Hillegersberg R, Roumen RM, et al. Neoadjuvant chemotherapy followed by surgery versus surgery alone in high-risk patients with resectable colorectal liver metastases: the CHARISMA randomized multicenter clinical trial. BMC cancer. 2015;15:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groot Koerkamp B, Sadot E, Kemeny NE, Gonen M, Leal JN, Allen PJ, et al. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017:JCO2016718346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahbari NN, Reissfelder C, Schulze-Bergkamen H, Jager D, Buchler MW, Weitz J, et al. Adjuvant therapy after resection of colorectal liver metastases: the predictive value of the MSKCC clinical risk score in the era of modern chemotherapy. BMC cancer. 2014;14:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. The Lancet Oncology. 2013;14(12):1208–15. [DOI] [PubMed] [Google Scholar]
- 18.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. The New England journal of medicine. 2005;352(7):734–5. [DOI] [PubMed] [Google Scholar]
- 19.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. [DOI] [PubMed] [Google Scholar]
- 20. https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/startpagina_-_crc.html.
- 21.Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. Mol Cancer Ther. 2009;8(5):1015–25. [DOI] [PubMed] [Google Scholar]
- 22.Buisman FE, Grunhagen DJ, Homs MYV, Grootscholten C, Filipe WF, Kemeny NE, et al. Adjuvant Hepatic Arterial Infusion Pump Chemotherapy After Resection of Colorectal Liver Metastases: Results of a Safety and Feasibility Study in The Netherlands. Annals of surgical oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buisman F, van der Stok E, Galjart B, Vermeulen P, Allen P, Balanchandran V, et al. Histopathological growth patterns as a guide for adjuvant systemic chemotherapy in patients with resected colorectal liver metastases. European Journal of Surgical Oncology. 2019;45(2):e10. [Google Scholar]
- 25.Resche-Rigon M, White IR, Bartlett JW, Peters SA, Thompson SG, Group P-IS. Multiple imputation for handling systematically missing confounders in meta-analysis of individual participant data. Stat Med. 2013;32(28):4890–905. [DOI] [PubMed] [Google Scholar]
- 26.C DR. Regression Models and Life-Tables. Journal of the Royal Statiscial Society. 1972;34(2):187–220. [Google Scholar]
- 27.Harrel FE. Regression Modeling Strategies: with applications to linear models, logistic and ordinal regression, and survival analysis: Springer; 2015. [Google Scholar]
- 28.S. EW Clinical prediciton models: a practical approach to developmentm validation and updating. New York: Springer; 2010. [Google Scholar]
- 29.Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC, Harrell FE Jr., van Klaveren D. Graphical calibration curves and the integrated calibration index (ICI) for survival models. Stat Med. 2020;39(21):2714–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of surgery. 1999;230(3):309–18; discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631–60. [DOI] [PubMed] [Google Scholar]
- 33.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29(1):89–102. [DOI] [PubMed] [Google Scholar]
- 34.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Annals of surgery. 2008;247(1):125–35. [DOI] [PubMed] [Google Scholar]
- 35.Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19(1):72–84. [DOI] [PubMed] [Google Scholar]
- 36.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. Journal of the American College of Surgeons. 1999;189(3):291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Wang K, Han Y, Liang JY, Li YH, Xing BC. Nomogram predicted disease free survival for colorectal liver metastasis patients with preoperative chemotherapy followed by hepatic resection. Eur J Surg Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 38.Kanemitsu Y, Kato T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer. World J Surg. 2008;32(6):1097–107. [DOI] [PubMed] [Google Scholar]
- 39.Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, et al. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. Br J Surg. 2014;101(7):856–66. [DOI] [PubMed] [Google Scholar]
- 40.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Annals of surgery. 2007;246(2):183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy SK, Kattan MW, Yu C, Ceppa EP, de la Fuente SG, Fong Y, et al. Evaluation of perioperative chemotherapy using a prognostic nomogram for survival after resection of colorectal liver metastases. HPB (Oxford). 2009;11(7):592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margonis GA, Amini N, Buettner S, Kim Y, Wang J, Andreatos N, et al. The Prognostic Impact of Primary Tumor Site Differs According to the KRAS Mutational Status: A Study By the International Genetic Consortium for Colorectal Liver Metastasis. Annals of surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 43.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102(10):1175–83. [DOI] [PubMed] [Google Scholar]
- 44.Gholami S, Kemeny NE, Boucher TM, Gonen M, Cercek A, Kingham TP, et al. Adjuvant Hepatic Artery Infusion Chemotherapy is Associated With Improved Survival Regardless of KRAS Mutation Status in Patients With Resected Colorectal Liver Metastases: A Retrospective Analysis of 674 Patients. Annals of surgery. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goffredo P, Utria AF, Beck AC, Chun YS, Howe JR, Weigel RJ, et al. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J Gastrointest Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 46.Nierop PMH, Galjart B, Hoppener DJ, van der Stok EP, Coebergh van den Braak RRJ, Vermeulen PB, et al. Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis. 2019;36(2):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nierop PMH, Hoppener DJ, van der Stok EP, Galjart B, Buisman FE, Balachandran VP, et al. Histopathological growth patterns and positive margins after resection of colorectal liver metastases. HPB (Oxford). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20(12):1964–70. [DOI] [PubMed] [Google Scholar]
- 49.Kemeny NE, Chou JF, Boucher TM, Capanu M, DeMatteo RP, Jarnagin WR, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buisman FE, Homs MYV, Grunhagen DJ, Filipe WF, Bennink RJ, Besselink MGH, et al. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases - the multicenter randomized controlled PUMP trial. BMC cancer. 2019;19(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018;153(7):e180996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gagnière J, Dupré A, Gholami SS, Pezet D, Boerner T, Gönen M, et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Annals of surgery. 2020;271(1):147–54. [DOI] [PubMed] [Google Scholar]
- 53.Steyerberg EW. Clinical prediction models: A practical approach to development, validation, and updating (statistics for biology and health) Springer. New York. 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


