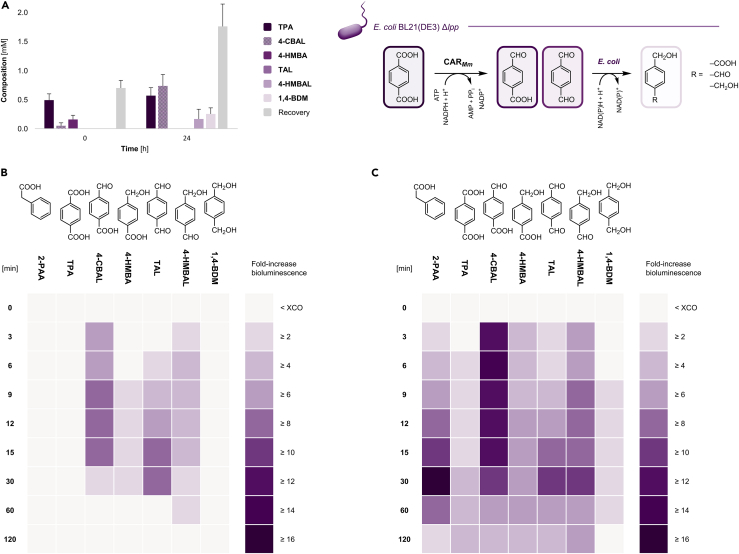
Figure 2.
Enzyme-coupled biosensor assembly in E. coli BL21(DE3) Δlpp
(A) CARMm reduces TPA to 4-CBAL and TAL, which are further reduced to 4-HMBA, 4-HMBAL, and 1,4-BDM by endogenous enzymes in vivo; PPTNi for posttranslational modification of CARMm is omitted for clarity. Experiments were performed in RCs of E. coli BL21(DE3) Δlpp (OD600 ≈ 10.0) co-expressing enzymes from pACYCDuet-1/carMm:pptNi (Bayer et al., 2021) in the presence of 2 mM TPA and 5% (ν/ν) DMSO as organic cosolvent. Sampling: 0 h (after the addition of TPA and mixing) and 24 h. Recoveries were reduced because of low solubility of TPA in resting cell medium (RCM) and the volatility of reaction compounds. GC yields are presented as mean values + standard deviation (SD) [mM] of biological replicates (n = 3); see also Figure S1.
(B) Direct detection of aldehydes (1 mM) by increasing bioluminescence over time in RCs of E. coli BL21(DE3) Δlpp expressing LuxAB from pLuxAB. (C) In situ production of aldehydes from carboxylates (1 mM) in RCs of E. coli BL21(DE3) Δlpp co-expressing LuxAB and CARMm/PPTNi; 2-phenyl acetic acid (2-PAA) was used as control. Experiments were performed in the presence of 1% (ν/ν) DMSO under HT assay conditions as described previously (Bayer et al., 2021); data presented as mean fold-increase bioluminescence obtained from biological replicates (n = 3). For results employing E. coli RARE, see Figure S3.