Abstract
OBJECTIVE
The objective of this study was to evaluate clinical features and response to deep brain stimulation (DBS) in G2019S LRRK2-Parkinson disease (LRRK2-PD) and idiopathic PD (IPD).
METHODS
The authors conducted a clinic-based cohort study of PD patients recruited from the Mount Sinai Beth Israel Genetics database of PD studies. The cohort included 87 participants with LRRK2-PD (13 who underwent DBS) and 14 DBS participants with IPD enrolled between 2009 and 2017. The baseline clinical features, including motor ratings and levodopa-equivalent daily dose (LEDD), were compared among LRRK2-PD patients with and without DBS, between LRRK2-PD with DBS and IPD with DBS, and between LRRK2-PD with subthalamic nucleus (STN) and internal segment of the globus pallidus (GPi) DBS. Longitudinal motor scores (Unified Parkinson’s Disease Rating Scale–part III) and medication usage were also assessed pre- and postoperatively.
RESULTS
Compared to LRRK2-PD without DBS (n = 74), the LRRK2-PD with DBS cohort (n = 13) had a significantly younger age of onset, longer disease duration, were more likely to have dyskinesia, and were less likely to experience hand tremor at disease onset. LRRK2-PD participants were also more likely to be referred for surgery because of severe dyskinesia (11/13 [85%] vs 6/14 [43%], p = 0.04) and were less likely to be referred for medically refractory tremor (0/13 [0%] vs 6/14 [43%], p = 0.02) than were IPD patients. Among LRRK2-PD patients, both STN-DBS and GPi-DBS targets were effective, although the sample size was small for both groups. There were no revisions or adverse effects reported in the GPi-DBS group, while 2 of the LRRK2-PD participants who underwent STN-DBS required revisions and a third reported depression as a stimulation-related side effect. Medication reduction favored the STN group.
CONCLUSIONS
The LRRK2-PD cohort referred for DBS had a slightly different profile, including earlier age of onset and dyskinesia. Both the STN and GPi DBS targets were effective in symptom suppression. Patients with G2019S LRRK2 PD were well-suited for DBS therapy and had favorable motor outcomes regardless of the DBS target. LRRK2-DBS patients had longer disease durations and tended to have more dyskinesia. Dyskinesia commonly served as the trigger for DBS surgical candidacy. Medication-refractory tremor was not a common indication for surgery in the LRRK2 cohort.
Keywords: deep brain stimulation, DBS, Parkinson’s disease, LRRK2, GPi, STN, functional neurosurgery
Pathogenic variants of the leucine-rich repeat kinase 2 (LRRK2) gene have been identified as a leading genetic cause of Parkinson disease (PD).1 The most common LRRK2 pathogenic variant is the glycine to serine substitution at position 2019 (G2019S), which is present in approximately 1% of patients with sporadic PD and 4% of patients with familial PD.2
Patients with G2019S LRRK2 parkinsonism (LRRK2-PD) develop a motor phenotype similar to those with idiopathic PD (IPD). This motor phenotype includes asymmetrical resting tremor, bradykinesia and rigidity, and a positive response to levodopa.3 LRRK2-PD patients are also at risk for developing motor fluctuations, dystonia, and bothersome dyskinesias.4 Group-wise clinical differences from IPD, however, have been identified. These differences include more frequent lower-extremity and gait impairment without associated cognitive impairment;4–6 furthermore, longitudinal analyses of LRRK2-PD suggest slower motor decline.7 Thus, many LRRK2 characteristics including levodopa responsiveness, motor fluctuations, and better cognitive performance align well with optimal candidacy for deep brain stimulation (DBS). Other features such as greater postural instability and gait disorder3 may augur a less positive response. How these factors influence DBS candidacy and response is not well described.
DBS can improve motor performance in patients with IPD, specifically alleviating the cardinal symptoms of tremor, rigidity, and bradykinesia and addressing motor fluctuations and dyskinesias.8–10 DBS is less efficacious for freezing of gait and balance disorders.11 The most common brain targets for PD are the subthalamic nucleus (STN) and the internal segment of the globus pallidus (GPi). Both targets have been shown to be safe and effective in IPD,12 although there is a paucity of target-specific information, especially as it relates to genotype.13
The objectives of the current study were to compare baseline clinical characteristics driving decisions to pursue DBS among G2019S LRRK2-PD. We also aimed to compare longitudinal motor and medication DBS outcomes between LRRK2-PD and IPD and to gather preliminary information about target-specific differences.
Methods
Participants
All participants were identified from the Genetics of PD Studies at Mount Sinai Beth Israel between July 2009 and July 2017. The main inclusion criteria included a diagnosis of IPD. Those harboring an LRRK2 G2019S pathogenic variant were classified as LRRK2-PD. Participants enrolled in Genetics of PD Studies without known pathogenic variants, classified as IPD, were recruited if they had undergone unilateral or bilateral STN or GPI DBS. Motor and nonmotor symptom data were systematically collected from medical records. Additional information was also obtained from medical records of the subset who underwent DBS surgery. IRB approval was received, and informed written consent was obtained from all patients.
Clinical Assessments
The assessments included: demographic information, Unified Parkinson’s Disease Rating Scale part I–IV (UPDRS- I–IV),14 Hoehn and Yahr stage, levodopa-equivalent daily dose (LEDD), and cognitive status as measured by the Montreal Cognitive Assessment (MoCA). Motor assessments were performed in the ON state, as defined by the clinician and patient, as part of routine clinical visits. Because postoperative motor evaluations in the OFF state were not systematically collected, motor OFF assessments were not included. Longitudinal assessments were obtained at multiple time points before and after surgery, with postoperative assessments at least 3 months after surgery to account for any microlesion effects. For subjects undergoing DBS, the following information was additionally and systematically extracted from the medical record: indication for DBS, date of surgery, brain target and rationale for target, implanting surgeon, and any surgical complications.
DBS Surgery
DBS eligibility and brain target choice were determined clinically through a multidisciplinary team discussion. Inclusion criteria for DBS eligibility were: presence of at least 2 cardinal motor features (resting tremor, bradykinesia, or rigidity), disease duration longer than 5 years, robust response to levodopa, persistent disabling symptoms such as motor fluctuations with troublesome OFF periods or dyskinesia despite optimal medical therapy, a minimum UPDRS-III OFF medication score of 25, stable medical therapy for at least 1 month prior to baseline, and the ability to comply with follow-up visits. Exclusion criteria were intracranial abnormalities contraindicating surgery, medical contraindications to surgery, clinical evidence of an atypical parkinsonian syndrome, active alcohol or drug abuse, pregnancy, dementia or significant cognitive impairment, or uncontrolled mood disorder. Surgical implantation of DBS electrodes was performed as previously described.15 In brief, all patients underwent stereotactic frame-based placement of bilateral DBS leads as well as microelectrode recording for target refinement. Procedures were performed by experienced functional neurosurgeons in New York or Florida. Once the optimal track was identified for implantation, the DBS lead was inserted. Lead location was confirmed using intraoperative CT (O-arm, Medtronic) merged with preoperative MRI/CT images. Postoperative programming and selection of optimal stimulation contacts were performed using clinical criteria.16
Statistical Analysis
All statistical analyses were completed using Stata (version 15, StataCorp LP). Univariate analyses of demographic variables were performed using chi-square test and Student t-test or nonparametric equivalents when necessary (Table 1). Linear mixed models adjusted by gender, age at PD onset, LEDD (as a time-dependent covariate), age at first visit or at surgery, and baseline UPDRS-III, allowing for subject-specific random intercepts to be used to compare UPDRS-III trajectories between LRRK2-PD and IPD groups. No corrections were made for multiple comparisons, as the analysis presented was deemed predominately descriptive and exploratory.
TABLE 1.
Demographic and baseline clinical characteristics of G2019 LRRK2 carriers, according to DBS status
| Clinical Characteristic | LRRK2 non-DBS (n = 74) | LRRK2 DBS (n = 13) | p Value |
|---|---|---|---|
| Male | 34/74 (45.95) | 9/13 (69.23) | 0.14 |
| Age at PD motor symptom onset, yrs | 61.63 ± 10.76 | 49.15 ± 11.31 | <0.001 |
| Age at PD diagnosis, yrs | 63.21 ± 10.86 | 50.62 ± 10.91 | <0.001 |
| Age at last non-DBS visit, yrs | 72.03 ± 10.36 | 63.08 ± 13.41 | 0.04 |
| Duration of disease, yrs | 9.75 ± 10.31 | 12.38 ± 6.27 | 0.04 |
| UPDRS-III score | 18.61 ± 13.18 | 18.30 ± 10.75 | 0.88 |
| Hoehn & Yahr score | 2.38 ± 1.03 | 2.25 ± 0.42 | 0.67 |
| Site of onset in leg | 35/67 (52.24) | 9/13 (69.23) | 0.36 |
| Tremor as presenting feature | 52/67 (77.61) | 5/13 (38.46) | 0.01 |
| MoCA score | 25.31 ± 3.63 (n = 52) | 24.43 ± 3.26 (n = 7) | >0.99 |
| UPDRS-I, question 1, intellectual impairment, score ≥1 | 32/56 (57.14) | 5/11 (45.45) | 0.52 |
| Dyskinesia ever present prior to DBS | 27/67 (40.3) | 13/13 (100) | <0.001 |
| Sustained levodopa response >5 yrs | 30/67 (44.78) | 11/13 (84.62) | 0.01 |
Data given as mean ± SD or number (%), unless otherwise indicated.
Results
Between July 2009 and July 2017, 94 participants with PD were identified who carried at least one copy of the G2019S pathogenic variant, 4 of whom were excluded for also harboring a GBA pathogenic variant. Demographic and clinical information was available for 87 LRRK2-PD participants, including 13 who had undergone DBS surgery (LRRK2-DBS). Of this subset, 4 LRRK2-PD participants had undergone GPi-DBS (3 bilateral and 1 unilateral) while 9 had undergone STN-DBS (8 bilateral and 1 unilateral). Of the 14 IPD-DBS patients identified with known genetic status, 2 had undergone GPi-DBS (1 bilateral and 1 unilateral) and 12 had undergone STN-DBS (11 bilateral and 1 unilateral; Supplementary Table 5).
Comparisons Among LRRK2-PD: DBS Versus No DBS
Univariate comparisons between 74 LRRK2-PD participants who had not undergone DBS (LRRK2-nonDBS) during the observation period and the 13 LRRK2-PD patients who had been treated with DBS are listed in Table 1. No significant differences were found in gender, baseline UPDRS-III score, Hoehn and Yahr stage, or baseline cognitive status (as measured by MoCA and UPDRS-I question 1). However, LRRK2-DBS participants had a younger age of onset (mean 49.2 ± 11.3 vs 61.6 ± 10.8 years, p < 0.001) and longer duration of disease at baseline (12.4 ± 6.3 vs 9.8 ± 10.3 years, p = 0.04). They were also less likely to have presented with hand tremor at onset (38.5% vs 77.6%, p = 0.01) and were more likely to have developed dyskinesia prior to DBS surgery (100% vs 40.3%, p < 0.001).
Longitudinal information was available for 32 LRRK2-nonDBS and 9 LRRK2-DBS individuals. LRRK2-nonDBS participants were seen an average of 6.2 times (range 1–15 times), whereas LRRK2-DBS participants were seen an average of 8.3 times (range 1–13 times), of which 3.8 visits (range 1–5 visits) took place prior to DBS surgery. An exploratory analysis was conducted on progression of symptoms. Prior to DBS, the LRRK2-DBS group progressed 0.02 UPDRS-III points per month, which was 0.10 points per month less rapidly than the LRRK2-nonDBS group (Fig. 1, Supplementary Table 1). This difference, however, was not found to be statistically significant and the sample size was small (odds ratio [OR] 0.10, 95% confidence interval [CI] −0.2 to 0.2, p = 0.93).
FIG. 1.
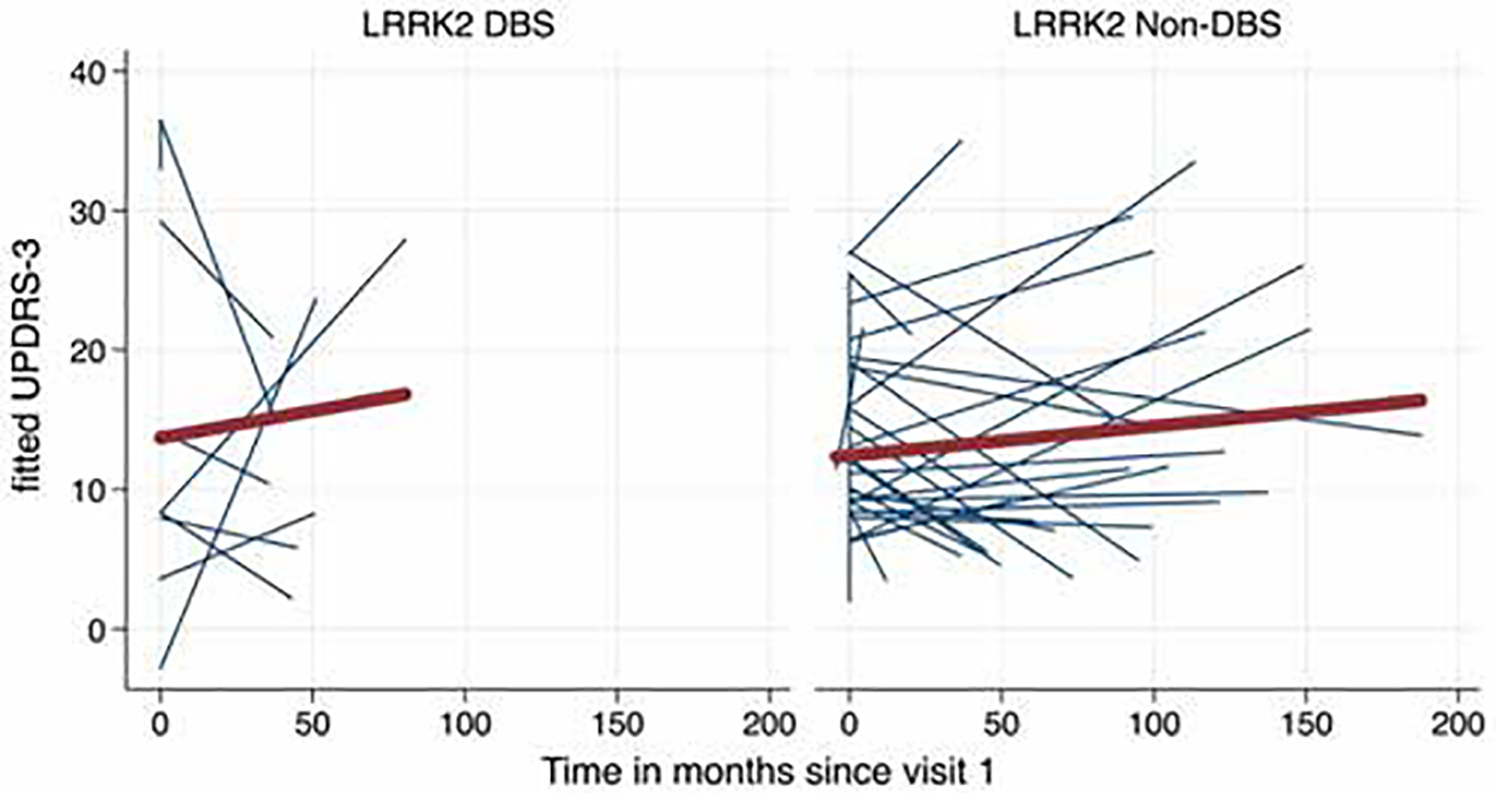
Spaghetti plot of fitted (predicted) motor trajectories (UPDRS-III) of LRRK2-PD subjects. Blue lines represent individual subjects and the red line is average trend of all subjects. Left: Preoperative motor trajectories of LRRK2-DBS subjects. Right: Preoperative motor trajectories of LRRK2 non-DBS subjects.
Comparisons Among Participants Undergoing DBS: LRRK2-PD Versus IPD
Baseline information was available for 9 LRRK2-DBS and 14 IPD-DBS participants (Table 2, Supplementary Table 1). LRRK2-DBS individuals had a significantly longer duration of disease at diagnosis than IPD-DBS patients (1.7 ± 1.2 vs 0.7 ± 0.7 years, p = 0.03), but the groups were otherwise comparable with similar gender distribution, age at diagnosis and disease onset, site of onset, duration of disease at surgery, UPDRS-III scores at baseline, and age at surgery. Additionally, there were no statistically significant differences between LEDD and median UPDRS-III scores (Table 2) at the time of surgery.
TABLE 2.
Demographic and baseline clinical characteristics of patients undergoing DBS, according to LRRK2 status
| Clinical Characteristic | IPD DBS (n = 14) | LRRK2 DBS (n = 9) | p Value |
|---|---|---|---|
| Male | 13/14 (92.86) | 7/9 (77.78) | 0.53 |
| Age at PD motor symptom onset, yrs | 52.33 ± 9.01 | 50.11 ± 13.56 | 0.72 |
| Age at PD diagnosis, yrs | 53.00 ± 9.12 | 51.11 ± 12.83 | 0.72 |
| Site of onset | |||
| Arm | 7/14 (50.00) | 3/9 (33.3) | 0.39 |
| Leg | 4/14 (28.57) | 3/9 (33.3) | >0.99 |
| Gait | 1/14 (7.14) | 3/9 (33.3) | 0.27 |
| Duration of disease at diagnosis, yrs | 0.67 ± 0.72 | 1.67 ± 1.22 | 0.03 |
| Disease duration at levodopa institution, yrs | 2.69 ± 1.93 | 6.25 ± 6.80 | 0.14 |
| Age at surgery, yrs | 64.1 ± 6.33 | 63.11 ± 15.09 | 0.97 |
| Duration of disease (diagnosis) at surgery, yrs | 10.59 ± 6.68 | 11.11 ± 5.13 | 0.52 |
| Duration of disease (symptoms) at surgery, yrs | 10.53 ± 6.27 | 12.78 ± 4.38 | 0.22 |
| Median LEDD at surgery (IQR) | 938 (498.5) | 1228 (491) | 0.09 |
| Median UPDRS-III score at surgery (IQR) | 9 (11) | 16 (21) | 0.13 |
Data given as mean ± SD or number (%), unless otherwise indicated.
The majority of individuals in both groups underwent bilateral DBS placement (11/13 in the LRRK2-DBS group and 12/14 in the IPD-DBS group), and the frequency of surgical complications and adverse effects were similar: 3/13 revisions for LRRK2-DBS patients versus 2/14 for IPD-DBS, 3/13 adverse effects for the LRRK2-DBS group versus 5/14 for IPD-DBS. However, LRRK2-DBS participants were significantly more likely to be referred for surgery because of severe dyskinesia (11/13 [85%] vs 6/14 [43%], p = 0.04) and were significantly less likely to be referred for medically refractory tremor (0/13 [0%] vs 6/14 [43%], p = 0.02) relative to IPD-DBS participants (Supplementary Table 5).
An exploratory longitudinal analysis of progression was performed. LRRK2-DBS and IPD-DBS participants had similar rates of motor worsening prior to surgery (Fig. 2 left, Supplementary Table 1), with IPD-DBS participants progressing an average of 0.10 UPDRS-III points per month less rapidly than LRRK2-DBS participants (95% CI −0.16 to 0.36, p = 0.47). Following surgery (Fig. 2 right, Supplementary Table 1), IPD-DBS participants worsened at a rate of 0.07 points per month more rapidly than did LRRK2-DBS participants (95% CI −0.16 to 0.02, p = 0.14). However, these differences in trajectories did not reach statistical significance.
FIG. 2.
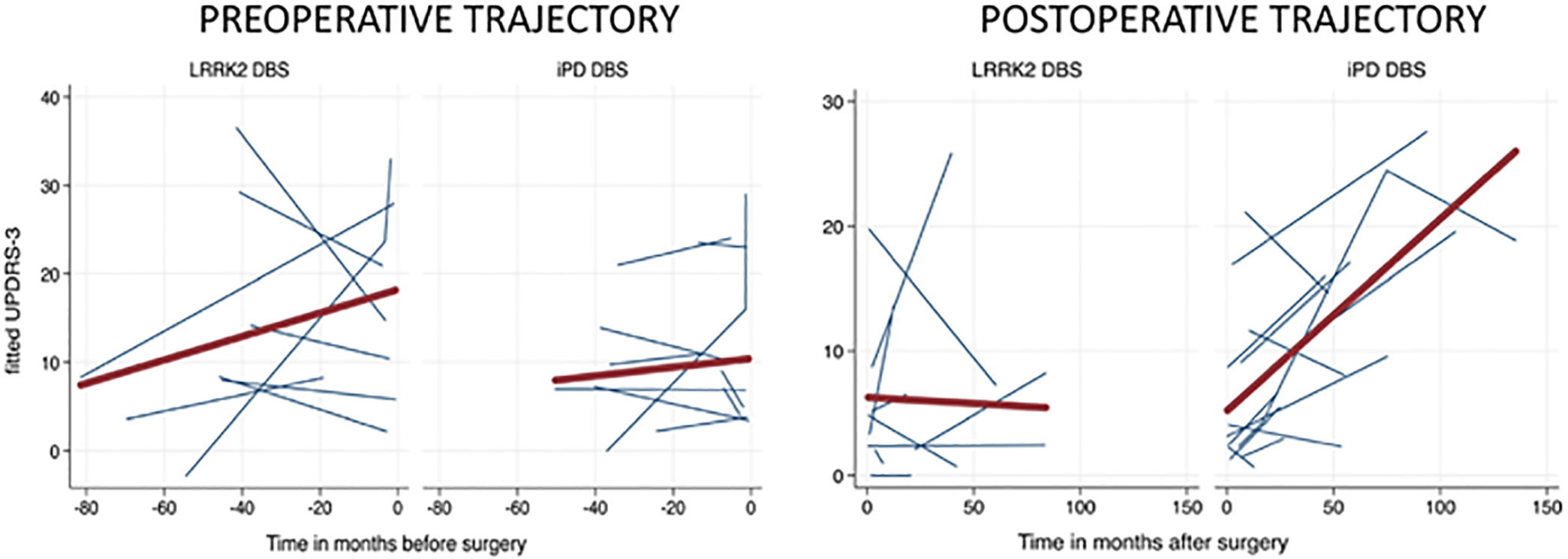
Spaghetti plot of fitted (predicted) motor trajectories (UPDRS-III) of LRRK2 and IPD subjects undergoing DBS. Blue lines represent individual subjects and the red line is average trend of all subjects. Left: Preoperative motor trajectories by LRRK2 status. Right: Postoperative motor trajectories by LRRK2 status.
Medication use was compared before and after surgery between LRRK2 and IPD cohorts (Supplementary Table 2). Both the LRRK2-DBS and IPD-DBS groups had significant reductions in medication dose with a median LEDD reduction of 378 units (interquartile range [IQR] 774 units) among LRRK2-DBS participants and of 487.5 units (IQR 655 units) among IPD-DBS participants (p = 0.02 for each).
Brain Target Choice Within the LRRK2-DBS Group
Four LRRK2-DBS participants underwent GPi-DBS while 9 underwent STN-DBS (Supplementary Table 5). Across both groups, the majority received bilateral electrode placement (3/4 [75%] for GPi-DBS and 8/9 [88%] for STN-DBS). There were no revisions or adverse effects reported in the GPi-DBS group, but 2 of the LRRK2-DBS participants who underwent STN-DBS required revisions and a third reported depression as a stimulation-related side effect. Indications for DBS were similar across groups, with the most common being motor fluctuations (3/4 [75%] in the GPi-DBS group and 7/9 [78%] in the STN-DBS group) and severe dyskinesia (3/4 [75%] in the GPi-DBS group and 8/9 [89%] in the STN-DBS group). Medication reduction was the primary rationale for target choice for all 9 of the STN-DBS patients, whereas the targets for each of the 4 GPi-DBS patients were chosen for different indications, including concern for cognition and significant dystonia.
Longitudinally, among the LRRK2-DBS cohort, those who underwent GPi-DBS had similar motor outcomes compared to those who underwent STN-DBS (Fig. 3). The LEDD was analyzed according to brain target. Overall, when combining groups, patients with STN-DBS had greater medication reduction (median 542 units, IQR 760 units, p = 0.004) than did those with GPi-DBS (median 238 units, IQR 524 units, p = 0.10). This effect was also present within the LRRK2-PD group, with significant LEDD reduction shown only among those with STN-DBS (p = 0.04; Supplementary Tables 3 and 4).
FIG. 3.
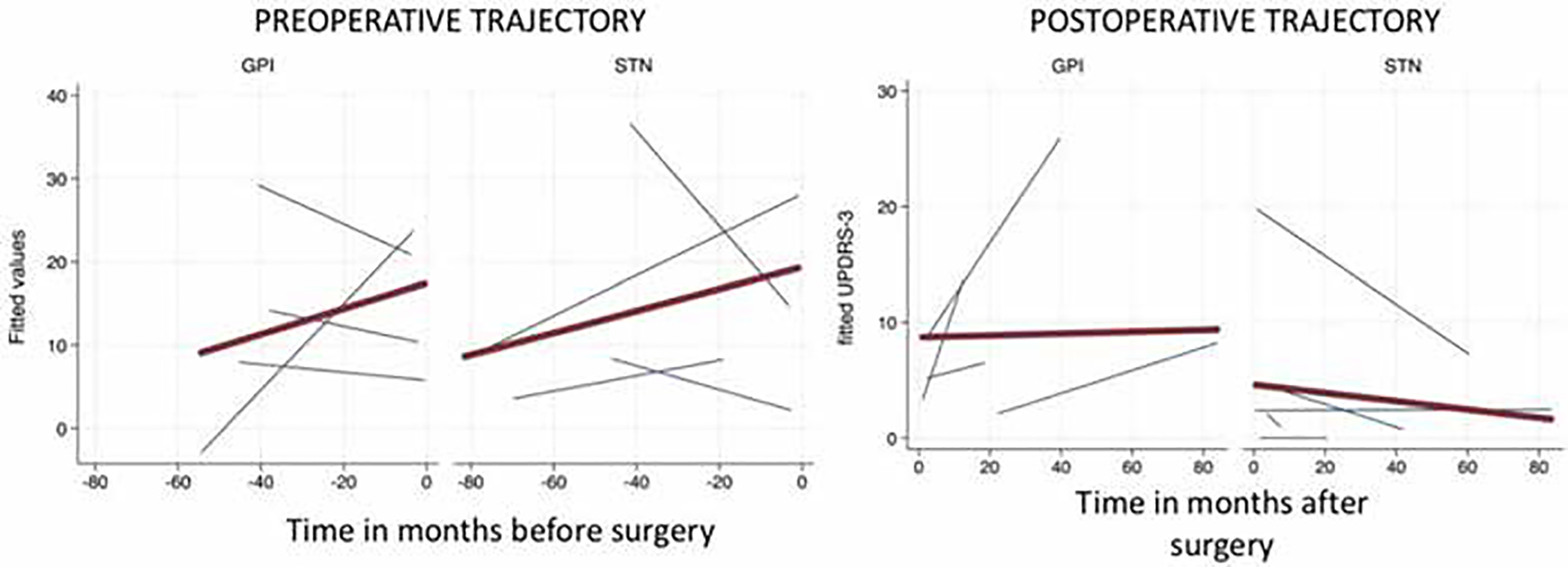
Spaghetti plot of fitted (predicted) motor trajectories (UPDRS-III) of LRRK2-PD subjects undergoing DBS. Blue lines represent individual subjects and the red line is average trend of all subjects. Left: Preoperative motor trajectories by brain target. Right: Postoperative motor trajectories by brain target.
Discussion
The current study characterized a cohort of patients with G2019S LRRK2 parkinsonism, with and without DBS, and collected preliminary outcome data on STN and GPi brain targets.
The cohort of 94 LRRK2-PD patients was relatively large and 13 were referred for DBS. Those referred for DBS had a distinct profile with a younger age of onset and longer duration of disease at the time of surgery (12.38 vs 9.75 years). The disease duration was longer than that observed in multiple randomized controlled DBS cohorts.17 This finding is consistent with prior studies showing slower LRRK2 progression7 and thus potentially presents a somewhat different, wider, optimum “window” for surgical intervention.17–23
The LRRK2-PD patients referred for surgery were less likely to present with hand tremor at disease onset (38% vs 78%). However, all LRRK2-DBS patients developed dyskinesia prior to DBS compared to only 40% of LRRK2-PD patients not referred for surgery. The causes for the excess of dyskinesia may be multifactorial and possible contributors include longer duration of disease and levodopa exposure. Regardless, the observation that the most common indication for surgical referral in LRRK2-PD patients was severe dyskinesia (85%) is an important one for the clinician. Another notable observation in this group is that medically refractory tremor did not occur in the LRRK2-DBS group and this information may be useful to the implanting team. Tremor is a commonly reported symptom in LRRK2-PD,2 but in our series it did not translate to a reason for proceeding to DBS surgery.
Although the sample size was small, our exploratory analysis of longitudinal progression in patients with LRRK2-PD who underwent DBS showed that these patients had a slightly slower rate of motor progression postoperatively compared to IPD-DBS patients. This improvement was observed for more than 2 years following surgery. Our findings should be interpreted with caution, but add to the growing body of literature favoring improved motor outcomes in G2019S LRRK2 PD compared to IPD.24,25
Our results demonstrate the safety and potential effectiveness of both STN and GPi-DBS in LRRK2-PD and are consistent with the two largest published studies on LRRK2 STN-DBS, which included 15 and 13 patients (all of whom had the G2019S pathogenic variant). Both of these previously published studies demonstrated improved motor outcomes for LRRK2-PD patients as compared to patients without pathogenic variants.25,26 In contrast, smaller studies have found either improvement or no difference in motor outcomes when comparing STN-DBS in LRRK2-PD to IPD.24,27,28
This is the first study to report on GPi-DBS outcomes in G2019S LRRK2 PD. The rationale for the GPi target choice in our cohort was concern for preserving cognition, mood, or the presence of significant dystonia. A few recent studies have suggested a slight worsening of cognition after STN-DBS,21,29 or a better effect on mood using GPi-DBS.30 More recent studies using different outcomes have shown conflicting results.31,32 GPi-DBS is, however, recognized for its strong antidyskinetic effects.33 Given that the majority of the LRRK2-DBS patients in this study were referred for DBS for severe dyskinesia, GPi-DBS may represent a reasonable and attractive brain target for LRRK2-PD with dyskinesia. Longitudinally, our GPi-DBS data demonstrated similar motor improvements to STN-DBS in UPDRS-III ON scores from 6 months to 2 years after surgery. The significant reduction in LEDD favored the STN-DBS group and this closely matched the results in other published cohorts.30,32 The number of surgical revisions and postoperative adverse effects was low in both cohorts and consistent with rates in IPD-DBS.21
This study has several limitations. The number of surgical subjects was small, which precluded confident interpretations of statistical analysis, especially regarding the brain target subgroups. Most, but not all, of the DBS procedures were performed by a single surgeon at a single site. The variability in site and surgeon may have also affected the DBS outcomes. Additionally, longitudinal data for motor outcomes were only available for ON UPDRS-III scores. A potential limitation of our study is the recruitment of all individuals from participants in genetics studies. Even among those not carrying known pathogenic variants, approximately half had a family history of PD, thus this comparison group may have not been as typical of a group with less likely genetic burden. However, if that was the case, it would have biased the results of our study toward the null hypothesis, and the found differences might have been more apparent had we compared them with a less genetic cohort.
Future larger longitudinal studies are warranted and should expand to examine cognitive and nonmotor outcomes, and quality of life. Lastly, our study was limited to one pathogenic LRRK2 genetic variant and investigating pathogenic and risk variants beyond G2019S will be needed but could be challenging, given even smaller numbers of available subjects.
Conclusions
Patients with G2019S LRRK2 parkinsonism are well-suited for DBS therapy and have favorable motor outcomes in both DBS targets. Among LRRK2-PD patients, those undergoing DBS have longer disease durations and tend to have more dyskinesia, and dyskinesia is commonly the trigger for DBS surgery. Medication-refractory tremor was not a common indication for surgery in our LRRK2 cohort.
Supplementary Material
Acknowledgments
This study was funded by the Edmond J. Safra Fellowship in Movement Disorders, NIH-NINDS grant nos. U01 NS107016–01A1 and K23NS0099441-O1A, the Empire Clinical Research Investigator Program, and the Bigglesworth Family Foundation.
Disclosures
Dr. Leaver was an Edmond J. Safra Fellow in Movement Disorders at Mount Sinai Beth Israel. Dr. Kopell serves as a consultant for Medtronic, Abbott, and ClearPoint Neuro. He receives funding from the NIH and the Michael J. Fox Foundation. Mr. Ortega is funded by the Bigglesworth Family Foundation. Dr. Okun serves as a consultant for the Parkinson’s Foundation, and has received research grants from the NIH, Parkinson’s Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and the UF Foundation. Dr. Okun’s DBS research is supported by NIH grant nos. R01NR014852 and R01NS096008. Dr. Okun is principal investigator of the NIH R25NS108939 training grant. He has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. He has participated in CME and educational activities on movement disorders sponsored by the Academy for Healthcare Learning, PeerView, Prime, QuantiaMD, WebMD/Medscape, Medicus, MedNet, Einstein, MedNet, Henry Stewart, American Academy of Neurology, Movement Disorders Society, and Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, Boston Scientific, Abbott, and Allergan, and the principal investigator has no financial interest in these grants. Dr. Okun has participated as a site principal investigator and/or co-investigator for several NIH, foundation, and industry-sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations. Ms. Elango is funded through the Empire Clinical Research Investigator Program. Dr. Bressman receives support for research from the Michael J. Fox Foundation. Dr. Saunders-Pullman receives grant support from NIH-NINDS U01 grant no. NS107016–01A1, NIH-NINDS U01 grant no. NS094148, the Bigglesworth Family Foundation, and the Michael J. Fox Foundation for Parkinson’s Research. Dr. San Luciano receives grant support from the NIH (NINDS grant no. K23NS0099441-O1A) and personal compensation for medico-legal consulting. Dr. Miravite reports being a consultant to Abbott and Medtronic.
ABBREVIATIONS
- CI
confidence interval
- DBS
deep brain stimulation
- GPi
internal segment of the globus pallidus
- IPD
idiopathic PD
- IQR
interquartile range
- LEDD
levodopa-equivalent daily dose
- MoCA
Montreal Cognitive Assessment
- OR
odds ratio
- PD
Parkinson disease
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Supplementary Tables 1–5. https://thejns.org/doi/suppl/10.3171/2021.7.JNS21190.
References
- 1.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lin-coln S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004; 44(4): 601–607. [DOI] [PubMed] [Google Scholar]
- 2.Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008; 7(7): 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia Santana H, Raymond D, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013; 28(14): 1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aasly JO, Toft M, Fernandez-Mata I, Kachergus J, Hulihan M, White LR, et al. Clinical features of LRRK2-associated Parkinson’s disease in central Norway. Ann Neurol. 2005; 57(5): 762–765. [DOI] [PubMed] [Google Scholar]
- 5.Alcalay RN, Mejia-Santana H, Mirelman A, Saunders-Pull-man R, Raymond D, Palmese C, et al. Neuropsychological performance in LRRK2 G2019S carriers with Parkinson’s disease. Parkinsonism Relat Disord. 2015; 21(2): 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcalay RN, Mejia-Santana H, Tang MX, Rosado L, Verbitsky M, Kisselev S, et al. Motor phenotype of LRRK2 G2019S carriers in early-onset Parkinson disease. Arch Neurol. 2009; 66(12): 1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders-Pullman R, Mirelman A, Alcalay RN, Wang C, Ortega RA, Raymond D, et al. Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol. 2018;75(3):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006; 355(9): 896–908. [DOI] [PubMed] [Google Scholar]
- 9.Obeso JA, Olanow CW, Rodriguez-Oroz MC, Krack P, Ku-mar R, Lang AE. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001; 345(13): 956–963. [DOI] [PubMed] [Google Scholar]
- 10.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009; 301(1): 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moro E, Schüpbach M, Wächter T, Allert N, Eleopra R, Honey CR, et al. Referring Parkinson’s disease patients for deep brain stimulation: a RAND/UCLA appropriateness study. J Neurol. 2016; 263(1): 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. [DOI] [PubMed] [Google Scholar]
- 13.St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75(14):1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003; 18(7): 738–750. [DOI] [PubMed] [Google Scholar]
- 15.Kochanski RB, Bus S, Pal G, Metman LV, Sani S. Optimization of microelectrode recording in deep brain stimulation surgery using intraoperative computed tomography. World Neurosurg. 2017;103:168–173. [DOI] [PubMed] [Google Scholar]
- 16.Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A. Programming deep brain stimulation for Parkinson’s disease: the Toronto Western Hospital algorithms. Brain Stimul. 2016; 9(3):425–437. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(suppl 14):S290–S304. [DOI] [PubMed] [Google Scholar]
- 18.deSouza RM, Akram H, Low HL, Green AL, Ashkan K, Schapira AH. The timing of deep brain stimulation for Parkinson disease in the UK from 1997 to 2012. Eur J Neurol. 2015;22(10):1415–1417. [DOI] [PubMed] [Google Scholar]
- 19.Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. [DOI] [PubMed] [Google Scholar]
- 20.Deuschl G, Schüpbach M, Knudsen K, Pinsker MO, Cornu P, Rau J, et al. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson’s disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat Disord. 2013; 19(1): 56–61. [DOI] [PubMed] [Google Scholar]
- 21.Weaver FM, Follett KA, Stern M, Luo P, Harris CL, Hur K, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012; 79(1): 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009; 65(5): 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11(2):140–149. [DOI] [PubMed] [Google Scholar]
- 24.Angeli A, Mencacci NE, Duran R, Aviles-Olmos I, Kefalopoulou Z, Candelario J, et al. Genotype and phenotype in Parkinson’s disease: lessons in heterogeneity from deep brain stimulation. Mov Disord. 2013;28(10):1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayad M, Zouambia M, Chaouch M, Ferrat F, Nebbal M, Bendini M, et al. Greater improvement in LRRK2 G2019S patients undergoing Subthalamic Nucleus Deep Brain Stimulation compared to non-mutation carriers. BMC Neurosci. 2016;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenbaum L, Israeli-Korn SD, Cohen OS, Elincx-Benizri S, Yahalom G, Kozlova E, et al. The LRRK2 G2019S mutation status does not affect the outcome of subthalamic stimulation in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):1053–1056. [DOI] [PubMed] [Google Scholar]
- 27.Schüpbach M, Lohmann E, Anheim M, Lesage S, Czernecki V, Yaici S, et al. Subthalamic nucleus stimulation is efficacious in patients with Parkinsonism and LRRK2 mutations. Mov Disord. 2007;22(1):119–122. [DOI] [PubMed] [Google Scholar]
- 28.Stefani A, Marzetti F, Pierantozzi M, Petrucci S, Olivola E, Galati S, et al. Successful subthalamic stimulation, but levodopa-induced dystonia, in a genetic Parkinson’s disease. Neurol Sci. 2013;34(3):383–386. [DOI] [PubMed] [Google Scholar]
- 29.Combs HL, Folley BS, Berry DT, Segerstrom SC, Han DY, Anderson-Mooney AJ, et al. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson’s disease: a meta-analysis. Neuropsychol Rev. 2015;25(4):439–454. [DOI] [PubMed] [Google Scholar]
- 30.Mansouri A, Taslimi S, Badhiwala JH, Witiw CD, Nassiri F, Odekerken VJJ, et al. Deep brain stimulation for Parkinson’s disease: meta-analysis of results of randomized trials at varying lengths of follow-up. J Neurosurg. 2018; 128(4): 1199–1213. [DOI] [PubMed] [Google Scholar]
- 31.Boel JA, Odekerken VJ, Schmand BA, Geurtsen GJ, Cath DC, Figee M, et al. Cognitive and psychiatric outcome 3 years after globus pallidus pars interna or subthalamic nucleus deep brain stimulation for Parkinson’s disease. Parkinsonism Relat Disord. 2016;33:90–95. [DOI] [PubMed] [Google Scholar]
- 32.Odekerken VJ, Boel JA, Schmand BA, de Haan RJ, Figee M, van den Munckhof P, et al. GPi vs STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology. 2016; 86(8): 755–761. [DOI] [PubMed] [Google Scholar]
- 33.Fan SY, Wang KL, Hu W, Eisinger RS, Han A, Han CL, et al. Pallidal versus subthalamic nucleus deep brain stimulation for levodopa-induced dyskinesia. Ann Clin Transl Neurol. 2020; 7(1): 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


