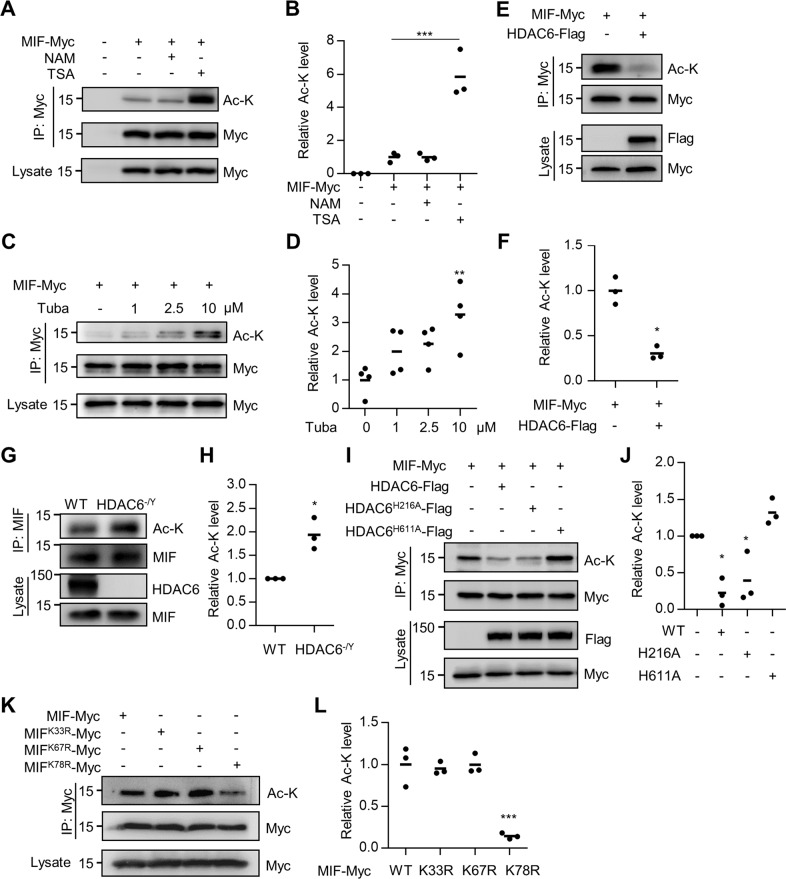
Fig. 1. Deacetylation of MIF protein by HDAC6.
A Increased MIF acetylation in cells treated with HDAC inhibitors. HEK293T cells were transfected with MIF-Myc and treated with nicotinamide (NAM) or trichostatin A (TSA). MIF protein was immunoprecipitated with anti-Myc antibody and probed with anti-Ac-K antibody to reveal acetylated MIF protein. B Quantification of MIF acetylation showed in A. Data shown were independent points and mean; one-way ANOVA; n = 3; ***p < 0.001. C Increased MIF acetylation in tubastatin A-treated cells. HEK293T cells were transfected with MIF-Myc and treated with HDAC6 inhibitor tubastatin A at indicated concentrations. MIF protein was immunoprecipitated with anti-Myc antibody and probed with anti-Ac-K antibody. D Quantification of MIF acetylation showed in C. Data shown were independent points and mean; one-way ANOVA; n = 4; **p < 0.01. E Decreased MIF acetylation in HDAC6-overexpressing cells. HEK293T cells were transfected with MIF-Myc with or without HDAC6-Flag. MIF protein was immunoprecipitated with anti-Myc antibody and probed with anti-Ac-K antibody. F Quantification of MIF acetylation showed in E. Data shown were independent points and mean; paired t-test; n = 3; **p < 0.01. G Increased MIF acetylation in HDAC6 mutant mice. The lysates from WT and HDAC6 mutant cortex were immunoprecipitated with anti-MIF antibody and probed with anti-Ac-K antibody. H Quantification of MIF acetylation showed in G. Data shown were independent points and mean; paired t-test; n = 3; *p < 0.05. I The second deacetylase domain of HDAC6 is required for MIF deacetylation. HDAC6-Flag, two deacetylase domain mutants (H216A and H611A), and MIF-Myc were transfected into HEK293T cells. MIF was immunoprecipitated with anti-Myc antibody and probed with anti-Ac-K antibody. J Quantification of MIF acetylation showed in I. Data shown were independent points and mean; one-way ANOVA; n = 3; *p < 0.05. K K78 of MIF is the major acetylation site. MIF-Myc, K33R-Myc, K67R-Myc, and K78R-Myc were transfected into HEK293T cells. MIF was immunoprecipitated with anti-Myc antibody and probed with anti-Ac-K antibody. L Quantification of MIF acetylation is shown in K. Data shown were independent points and mean; one-way ANOVA; n = 3; F = 27.39, p < 0.001; ***p < 0.001.