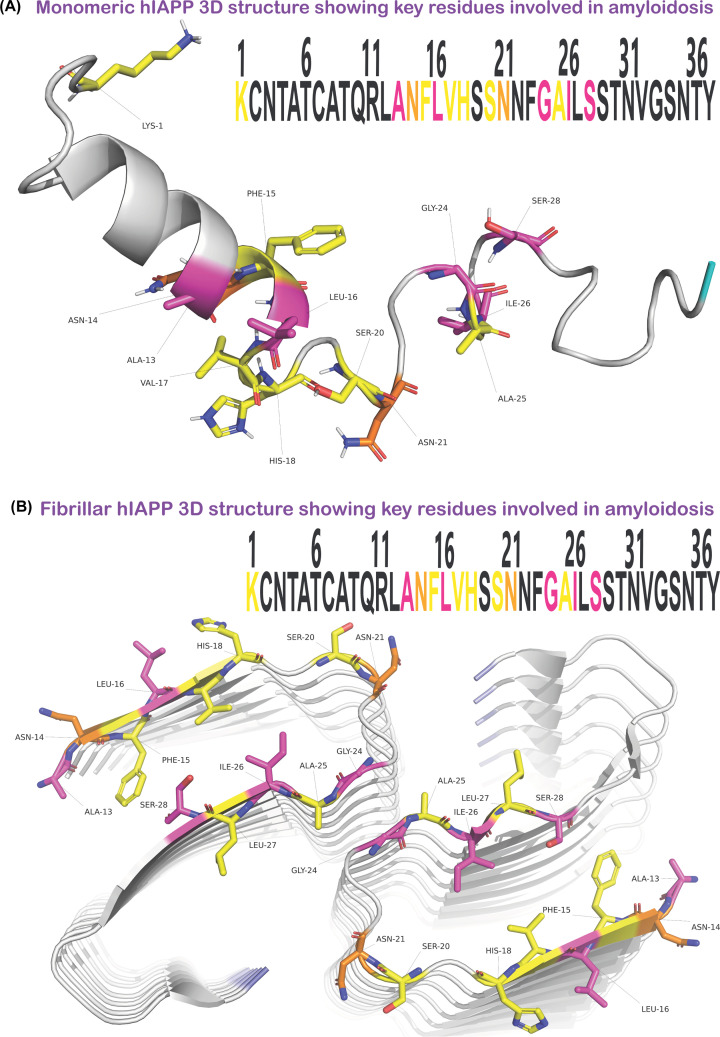
Figure 3. The identification of important residues linked to amyloidosis highlighted on solved 3D structures of hIAPP.
The colours highlight single residue mutations that abolish (purple), or accelerate (yellow) amyloid formation, or both (orange) and can be seen in the amino acid sequence within each figure. (A) Structure of recombinant hIAPP with an amidated C-terminal and oxidised cysteines, solved by solution NMR (PDB code = 5MGQ). (B) Structure of synthetic wildtype hIAPP peptide that is amidated at the C-terminal and aggregated in vitro to form fibrils and solved by cryo-EM (PDB code = 6Y1A). (A,B) are created using Pymol (http://www.pymol.org/pymol) and protein structures 5MGQ and 6Y1A were obtained from the Protein Data Bank (https://www.rcsb.org/).