Abstract
Use of three-dimensional bioprinting for the in vitro engineering of tissues has boomed during the past five years. An increasing number of commercial bioinks are available, with suitable mechanical and rheological characteristics and excellent biocompatibility. However, cell-laden bioinks based on a single polymer do not properly mimic the complex extracellular environment needed to tune cell behavior, as required for tissue and organ formation. Processes such as cell aggregation, migration, and tissue patterning should be dynamically monitored, and progress is being made in these areas, most prominently derived from nanoscience. We review recent developments in tissue bioprinting, cellularized bioink formulation, and cell tracking, from both chemistry and cell biology perspectives. We conclude that an interdisciplinary approach including expertise in polymer science, nanoscience, and cell biology/tissue engineering is required to drive further advancements in this field toward clinical application.
1. Introduction
Tissues and organs are three-dimensional by nature. However, for cost, convenience, and historical reasons, most biological features have been discovered in two-dimensional cell culture experiments. Meanwhile, the engineering of tissues and organs at the human scale is still far from reach for currently existing technologies. Three-dimensional approaches such as organoids or spheroids have revealed interesting aspects of cell biology (mainly at early developmental stages),1 but their small scale allows for drug screening and disease modeling only, not for organ replacement. As a result, pharmaceutical development pipelines (which mostly rely on preclinical results based on 2D cell culture and animal models) are highly inefficient due to elevated drug attrition rates.
In vitro tissue engineering has been around for several decades. Although the field abounds in notable preclinical advances, they have not resulted in changes in clinical practice—with few exceptions such as tissue-engineered skin, corneal epithelium substitutes, and autologous chondrocyte-laden scaffolds. Among other issues, the high complexity and cost of existing technologies have prevented widespread adoption of tissue engineering by the clinical and research communities. The advent of 3D bioprinting, i.e., of printers able to deposit cells in a controlled fashion, promised to democratize adoption of these novel technologies and even to resolve the shortage of human organs for transplantation. However, long-standing problems (such as oxygenation of large 3D structures) and emerging issues (such as cell compatibility with extrusion through narrow printing heads and the effect of chemical cross-linking on cellular behavior) await resolution. Similarly, the use of 3D bioprinting as an in vitro tissue modeling tool calls for the design of tailored bioinks that allow cell tracking within complex 3D structures, in turn posing novel challenges on the development of imaging, tracking, and bioink formulation tools.
In this mini-review, we address recent developments in tissue bioprinting, cell-laden bioink formulation, and cell tracking, from the perspectives of both chemistry and cell biology. We conclude that an interdisciplinary approach including expertise in polymer science, nanoscience, and cell biology/tissue engineering will be required to achieve the necessary advancements toward clinical application.
2. Tissue Bioprinting
Early 3D manufacturing started back in 1986 with a patent filed by Charles Hull on stereolithography.2 The possibility of using additive manufacturing on biological components or biomaterials (bioprinting) by extrusion or inkjet printing technologies kick-started a race for the development of bioinks, i.e., biomaterials that are suitable as inks for the bioprinting process. Currently, a broad catalogue of bioink formulations is commercially available. Notwithstanding, in-house production is still widespread because it involves lower cost and offers increased tailoring opportunities.
Hydrogels are soft, biocompatible, and often biodegradable materials amenable for the inclusion of cells. Because of their high water content, they can absorb up to thousands of times their dry weight in water, and because of their viscoelastic properties, they resemble living tissues and, thus, are ideal candidates for the formulation of bioinks.3 Moreover, depending on their composition, they can also respond to external stimuli such as pH, temperature, light, pressure, etc. Protein-based, polysaccharide-based, synthetic, and decellularized extracellular matrix (dECM)-derived hydrogels have been employed for bioink formulation. These biomaterials can be printed as single components, and the resulting scaffolds can be cellularized (i.e., seeded with cells) postprinting, under standard in vitro culture conditions. In this case, harsh extruding and cross-linking conditions may be employed since the bioprinting process is not constrained by live cells. Alternatively, cell-laden hydrogels or bioinks can also be used to directly print 3D tissues with controlled cell density, either through cell suspension printing or spheroid printing, to better mimic a physiological architecture. Oxygenation of 3D printed constructs has also been envisaged, as a means to ensure cell survival, thereby improving the metabolic activity and cell viability under hypoxic conditions. Additionally, cell behavior can be fine-tuned by exposing the cells to biologically active molecule-loaded inks or by adding any dynamic aspect to the three spatial dimensions of printing. This is sometimes referred to as “4D bioprinting”.
3. Current Trends in Bioink Formulation
The chemical composition of hydrogels used for bioprinting has a strong impact on the mechanical properties of the printed construct. Nowadays, commercial “starter” kits enable the preparation of hydrogel-based bioinks that emulate the mechanical requirements of the tissue of interest. However, no single material will fully resemble every feature of a target tissue, mechanical or otherwise. Thus, bioink tailoring and modification are prominent in the literature.4 The lack of standardization in bioink formulation results in batch-to-batch variation and increased interlab variability in research outcomes.
Use of monomaterial bioinks based on biomaterials with a long tradition in tissue engineering is commonplace, although some meaningful differences in usage may be inferred from the literature (Figure 1). Of note, each material will have both positive and negative properties to be considered (Table 1). Other aspects that should be contemplated when modifying these materials for extrusion printing include shear-thinning properties and cross-linking dynamics.5 As monomaterials rarely fulfill all of the requirements needed to mimic native ECMs, they are often used in combination to improve their printability and biological significance.6 Still, their modification results in interesting hydrogels, and many such bioinks are commercially available. Within this section, we will further expand on two trending bioink formulations: the use of extracellular matrix from decellularized organs and nanocomposites.
Figure 1.
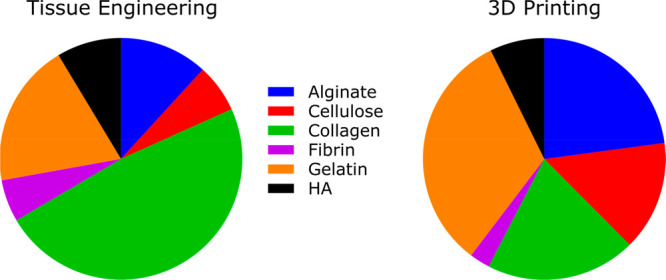
Distinct use of biomaterials in the tissue engineering and 3D printing fields. A PubMed search with the terms “Tissue Engineering” and “3D Printing” was conducted on February 1, 2022, for all articles published in 2021. A total of 19 542 and 4624 articles were found, respectively. Within the primary search results, mention in the title and abstract text of the following biomaterials was assessed: “Alginate”, “Cellulose”, “Collagen”, “Fibrin”, “Gelatin”, and “Hyaluronic acid” (HA). A total of 3566 articles (18.2%) in the “Tissue Engineering” category and 660 articles (14.3%) in “3D printing” quoted at least one of these biomaterials. The pie charts show the proportion of articles mentioning each of the biomaterials within each category, color-coded as follows: Alginate (blue), Cellulose (red), Collagen (green), Fibrin (violet), Gelatin (orange), HA (black). Alginate, cellulose, and gelatin (i.e., denatured collagen) seem to be used more prominently in 3D printing, while native, nondenatured collagen is prominently used in tissue engineering applications.
Table 1. Pros and Cons of Commonly Used Mono-Material-Based Inks.
| biomaterial | pros | cons | refs |
|---|---|---|---|
| alginate | - High similarity with polysaccharides in the native human extracellular matrix (ECM) | - It often requires modifications to guarantee cell attachment | (6), (18), (19), (20) |
| - Excellent biocompatibility and gelation properties | - Relatively inert for mammalian cells | ||
| - Excellent for cell encapsulation | |||
| - Versatile viscosity | |||
| collagens | - Family of structural proteins abundantly found in the ECM | - Slow gelation dynamics | (20), (21), (22), (23) |
| - Availability, thermosensitive properties, and good viscosity of collagen solutions, particularly for collagen type I | - Poor rheological properties | ||
| - Limited mechanical properties of the scaffolds | |||
| fibrin | - Common in wound healing applications | - Fast degradation | (6), (23), (24) |
| - Excellent biocompatibility | - Requires cross-linking | ||
| - Biodegradable and nonimmunogenic | |||
| - Induces cell attachment, proliferation and ECM formation | |||
| gelatin | - Forms thermosensitive gels, mainly composed of denatured collagen | - Unstable at temperatures required for cell culture | (4), (6), (12), (19), (21),23 |
| - Easily moldable by temperature or UV radiation if modified with methacrylate | - Limited mechanical properties of the scaffolds | ||
| - Widely used in tissue engineering, especially in disease modeling | |||
| hyaluronic acid (HA) | - Glycosaminoglycan (GAG) present in the ECM, with excellent hydration properties | - Unstable, it degrades fast | (18), (25) |
| - Very soft biomaterial | - In its pure state, it does not provide structural support | ||
| - Cross-linkable by UV radiation if modified with methacrylate |
3.1. Decellularized Extracellular Matrix (dECM)-Based Bioinks
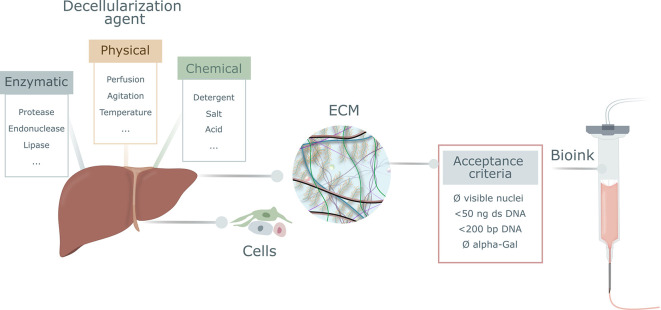
The microenvironmental niches of cells within tissues and organs are extremely complex, and relatively small modifications in their composition may have a major impact on cell behavior. Moreover, tissues and organs are dynamic in their response to extracellular cues, and the complexity and uniqueness of each tissue’s ECM at a given time point are far beyond current engineering capabilities. Tissue engineers are making increasing use of hydrogels based on decellularized extracellular matrix (dECM), to better mimic native cellular microenvironments. The methods currently used to remove cells while preserving the ECM structure can be classified as (i) enzymatic, (ii) chemical, and (iii) physical. The decellularization process must strike a balance between optimal removal of cell material and minimal damage to ECM integrity. For the former, minimal acceptance criteria are commonly used, such as having <50 ng of dsDNA per mg dry weight and DNA fragments <200 bp (Figure 2).7 For the latter, establishing adequate protocols and ensuring a thorough characterization of the resulting material are key to knowing the effects of the agents in the ECM, even though the native architecture will be unavoidably altered during the required digestion steps in the dECM ink formulation process.
Figure 2.
Commonly used strategies for organ decellularization and acceptance criteria for decellularized extracellular matrix (dECM). A number of enzymatic, physical, and chemical agents can be used to remove cells from organs and to obtain purified extracellular matrix (ECM), which can be used as a starting material for bioink formulation. Commonly used acceptance criteria to establish full decellularization of the tissue are the absence of visible nuclei in sections fluorescently stained for nucleic acid detection (DAPI or similar); the presence of less than 50 ng of double-stranded DNA (dsDNA) per mg dry weight, with fragments below 200 base pairs (bp); and the absence of the alpha-Gal epitope (Galalpha1–3Galbeta1-(3)4GlcNAc-R) of nonprimate mammals.
When dECM is sourced from nonprimate mammals, the lack of alpha-gal expression (an epitope present in mammalian cells but not in humans) is commonly studied. An early assessment found abundant contaminating cell traces in commercially available “acellular” matrices, even in those being used in the clinic.8 Nowadays, the situation has probably improved, but not much data is available so far.
Animal-sourced dECM-based bioinks have a relevant advantage over human materials: they can be readily obtained from waste tissue (for instance, from the food industry) and present excellent biocompatibility, which facilitates cell growth after printing. However, they entail several drawbacks that should be accounted for when choosing any material. Their drawbacks are related to the low viscosity or poor consistency that translate into a limited printability. On its part, human dECM may be troublesome because of sourcing and logistics issues. In all cases, biological safety must be ensured to prevent transmissibility of infectious agents (as usually tested for all types of transplantation).
The two main macromolecular components of the ECM are glycosaminoglycans (GAGs) and fibrous proteins. The GAG molecules are negatively charged—therefore water attractants—and are usually bound to proteins, forming proteoglycans. This process ensures hydration of the ECM and endures compressive forces. The fibrous matrix proteins (collagen, laminin, and elastin, among others) organize the ECM structure, thus providing resistance to tensile forces. The material obtained from solubilizing decellularized tissue is usually thermo-cross-linkable, as a consequence of the preponderance of collagen, which is thermoresponsive. This property entails significant variations of rheological properties during the printing process if the temperature is not controlled and constant, thereby leading to less reproducible printed structures due to the heterogeneity of the biochemical composition of the extruded fluid. As printing without any modification is often difficult, the use of photosensitive groups or temperature controlling modules to quickly harden the material after printing has been suggested to improve both the rheological properties and printability of dECM-based bioinks.9
On the other hand, dECM-based bioinks preserve remarkably well the native composition of the ECM, including key factors absent in commercial bioink formulations. Additionally, their complex biochemical composition may induce the synthesis of new ECM by the cells in the scaffold.10
As a meaningful application, a good number of dECM-based clinical implants are commercially available, most of them originating from porcine dermis, and some also being converted into bioinks. Such implants are used in clinical repair processes such as wound healing. Although basement membrane-like extracts such as Matrigel are commercially available and can be printed, they are mostly based on tumor-derived ECM, which can be troublesome. Notwithstanding, these in vitro generated ECM extracts are widely used in in vitro cell migration and differentiation assays, and an increased use in bioprinting applications can be foreseen. In our opinion, dECM-based bioinks must be carefully chosen. In an analogy from the past, a lot of cell biology findings were based on experiments done on immortalized cell lines. The use of 2D cultures of primary cells has demonstrated that plenty of the assumptions were simply wrong because altered tumoral biology does not resemble homeostatic (physiological) cell responses. Perhaps a parallel could be drawn here to state that the use of dECM from healthy tissue will replicate best the native tissue biology. The use of dECM from highly regenerative or highly responsive/adaptive organs, such as umbilical cord or fat tissue, may promote some of the characteristics needed for reignition of the regenerative potential in damaged areas.
3.2. Nanocomposite Bioinks
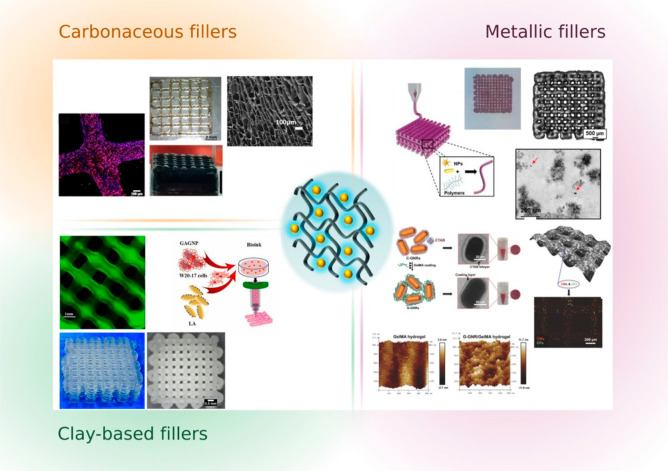
Within the diverse recent trends in biofabrication, polymer functionalization, nanocomposites, and supramolecular bioinks are worth mentioning. Composite bioinks have been obtained from the combination of hydrogel biomaterials and nanomaterials, for biomedical engineering. Nanoparticles, such as gold nanoparticles, can impart specific properties to the bioink such as optical, mechanical, thermal, etc., resulting in bioinks with advanced features. Examples include the enhancement of electrical conductivity, printability, or mechanical properties of the inks, as well as modulation of shear-thinning during extrusion.11 Nanosilicates, nanocellulose, or hydroxyapatite nanoparticles have also been used as ink-reinforcing agents3,12−14 (Figure 3).
Figure 3.
Schematic representation of nanocomposite hydrogels. Hydrogel-based inks can be reinforced with different types of nanofillers such as carbonaceous fillers for conductive properties (graphene, carbon nanotubes, etc.) (adapted from ref (13). Copyright 2021 Elsevier), metallic nanoparticles such as gold nanorods for optical or heating properties (adapted from ref (12). Copyright 2017 John Wiley & Sons and ref (18). Copyright 2020 John Wiley & Sons), or clay-based fillers as mechanical reinforcements (laponite, montmorillonite, silica, etc.) (adapted from refs (3, 14). Copyright 2021 Elsevier). The choice of the nanoparticles is driven by the desired final properties of the printed 3D models.
The modification of inks with NPs can also be directed toward improved imaging, sensing, or drug delivery. These applications are being gradually incorporated to the bioprinting field. However, in many cases structure–property–function relationships remain to be elucidated.
4. Bioink Characterization and Scaffold Testing
Several characterization tests must be performed at different stages of the bioprinting process, to ensure that critical quality requirements are met.
4.1. Rheology
In order to optimize bioink printability, rheological measurements should be carried out, to gain insight into the viscoelastic properties of the materials and other potential behaviors, such as shear thinning, shear thickening, and their relation to strain rate.5 An ideal bioink should be formulated so that it can be deposited in a controlled manner, regardless of the printing method. In the case of extrusion-based printers, it is crucial that the chosen bioink exhibits shear-thinning features; i.e., it flows under an applied force (shear) but maintains the shape when the force is removed (viscoelasticity). To this effect, rheology modifiers such as thickeners or high molecular weight molecules, such as nanocellulose or clays, are commonly used with the purpose of increasing viscosity as well as shear-thinning properties.
4.2. Printing Fidelity
In the particular case of extrusion bioprinting, the rheology and extrudability of the ink are as important as the filament properties. However, as a general rule, filament formation, uniformity of the printing filament or the collapse of the printed construct are quantifiable parameters that must be analyzed and tuned to obtain high quality scaffolds.5 Furthermore, the geometry of the pores as well as the filament circularity should also be assessed.
4.3. Scaffolds: Mechanical and Stability Studies
Once a bioink formulation is optimized and after printing a predesigned structure, mechanical tests are required to evaluate the integrity and mechanical properties of the scaffold. Depending on the application of the printed structure, uniaxial tension, compression, indentation, or dynamic mechanical tests are typically carried out to confirm that the printed scaffold fulfills the mechanical requirements of the targeted tissue. In the particular case of hydrogels, it is important to assess the swelling behavior. Hydrogels can absorb several times their weight in water, and the medium uptake depends on their chemical nature and cross-linking degree. Another fundamental cue is the degradation of the construct, which can be tailored to the intended application of the material. If tissue replacement is desired, cells are expected to secrete their own ECM while degrading the ECM in the construct. Nonetheless, bioink-based hydrogels should be stable and show a controlled degradation under cell culture conditions, in the case of in vitro models for high-throughput analysis.
4.4. Biocompatibility
In most published studies, biological suitability of bioinks is executed solely through biocompatibility tests. A number of indirect viability/biocompatibility standard tests are described in the ISO 10993 standards for the biological evaluation of medical devices.15 Such tests provide complementary results on the biocompatibility of the materials, helping the researcher determine whether they are suitable for cell culture, for implantation into an organism, or simply not biocompatible. Thus, assessment of cellular viability is essential for cellularized bioinks, as they are exposed to diverse factors that may compromise their integrity. However, an often-neglected aspect in the literature is that, besides viability, the bioprinting process may also affect cell phenotype and functionality. The latter must be tested, ideally during and after scaffold maturation in tissue culture, as well as after implantation into relevant animal models.
4.5. Cell Tracking and Scaffold Imaging
Cells are live, changing entities, which constantly migrate and interact with their microenvironment, which is thus continuously modified. Therefore, tracking bioprinted models along both space and time, is required to identify the best window for transplantation of cellularized scaffolds. Regarding imaging, most laboratories use confocal laser scanning microscopy (CLSM) as the main tool, which is based on a laser beam being focused into the specimen, where it excites fluorescent molecules. The emitted fluorescence is then collected by the objective lens and focused by a pinhole, which blocks fluorescence emission from above or below the focal plane. Imaging over a particular “z” plane is particularly interesting for 3D printing because bioprinted scaffolds are deposited layer-by-layer. However, excitation of the fluorescent dyes may compromise sample integrity, meaning that CLSM is limited for prolonged follow-up experiments.
Therefore, the design of increasingly complex 3D printed models should be accompanied by the development of analytical and imaging tools that can monitor cell evolution over long periods of time while preserving the integrity of the cell-laden scaffold. In this direction, surface-enhanced Raman spectroscopy (SERS) has arisen as a relevant tool that ensures high penetration depth in 3D and high selectivity, while avoiding commonly encountered problems of CLSM such as photobleaching.
SERS is a nondestructive technique that reveals the vibrational fingerprint of target molecules interacting with plasmonic metal NPs. The efficiency of the signal enhancement can be tuned if the excitation wavelength of the laser that irradiates the sample matches the surface plasmon resonance of the NPs. Apart from the detection of “free” molecules such as biomarkers, SERS can be used like CLSM for live-cell imaging with the help of so-called SERS nanotags.16 In this case, the plasmonic NPs are decorated with Raman reporter (RaR) molecules. As each RaR will have its unique fingerprint, SERS nanotags can be employed to label different cell types, which can subsequently be imaged with a single laser source, leading to an excellent multiplexing capacity of the technique (Figure 4). Moreover, the excitation wavelength in SERS can be shifted to the near-infrared (NIR) region where light penetration through the biological tissue is optimal. By following this approach, SERS imaging over the “z” plane of more complex 3D cell models or tissues using a multilayered cell model consisting of alternating layers of SERS nanotag-labeled cells has recently been reported.17
Figure 4.
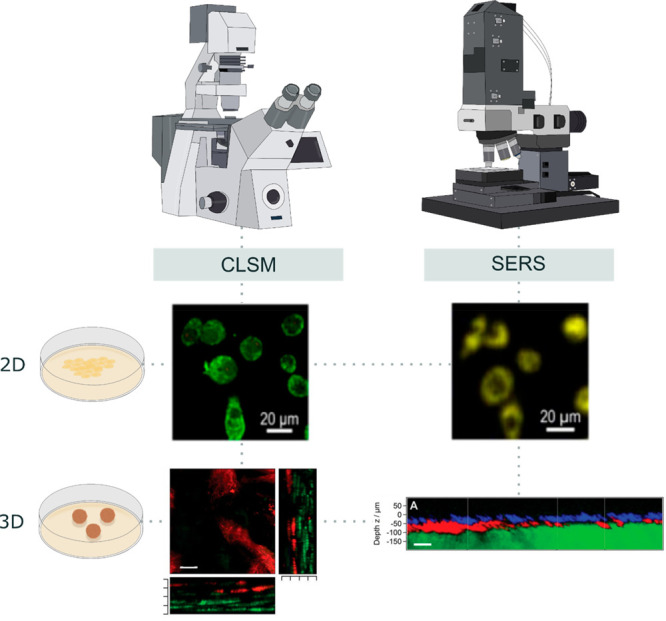
Cell imaging by confocal laser scanning microscopy vs surface-enhanced Raman spectroscopy. CLSM requires from fluorescent labeling of the samples and has been traditionally used to image 3D cell cultures or tissues, but it can also image organelles at single-cell resolution. Besides the detection of various molecules, SERS can also serve as an imaging technique for cells labeled with SERS nanotags. Both 2D and 3D live-cell cultures can be imaged, guaranteeing high penetration through the sample in a less destructive way. Likewise, multiplexing is feasible with either of the aforementioned techniques, highlighting their versatility. Adapted from ref (16). Copyright 2020 American Chemical Society, and ref (17). Copyright 2020 John Wiley & Sons.
The adaptability of the plasmonic nanoparticle surface chemistry makes them ideal for incorporation into hydrogel-based bioink formulations. In the case of gold nanoparticles, their biocompatibility also makes them suitable for the preparation of nanocomposite bioinks. As an example, gelatin-based nanocomposite bionks have been successfully prepared through the incorporation of gold nanorods, to achieve functional cardiac tissue.12 Recent works highlight the potential of SERS as both a detection tool in 3D-printed scaffolds and an imaging technique for complex cell models, which should be soon extended to the 3D printing of cellularized bioinks.18
Being able to decipher the underlying mechanisms of cell migration and differentiation by means of 3D culture modeling would contribute to a better understanding of both health and disease tissue physiology, as well as to a faster development of diagnostic and therapeutic tools.
5. Four-Dimensional Bioprinting
As mentioned above, 4D bioprinting aims to mimic in vivo biological functionality to the greatest extent. It comprises the incorporation of “time” as a fourth dimension to 3D bioprinting, with no need for additional equipment; there is however no consensus in the definition of the temporal feature. The most extended practice is the design of inks containing smart biomaterials that respond to one or multiple stimuli, once printed. The nature of such stimuli can be physical (such as light, temperature, magnetism, or cellular traction), chemical (pH or ionic strength), or biological (glucose, enzymes). These events will trigger changes in the conformation, size, and/or functionality of the bioink. Considering that no tissue presents a homogeneous composition in terms of ECM, phenotype, or mechanical properties, 4D bioprinting may be needed to generate the complex gradients that guarantee a functionality similar to that of native tissues.
The design of smart bioinks must thus pay close attention to stiffness and biocompatibility, parameters that do not usually go together. Computational models may help in defining scaffold structures, predicting their evolution, and foreseeing the effect of applying one or more stimuli to them. Concomitantly, the search for smart bioinks is motivating the development of composite bioinks, such as nanomaterial-based designs. Their ability to react toward diverse stimuli favors their use for the controlled release of encapsulated drugs, incorporation of growth factors, or vessel formation studies.
6. Concluding Remarks
Despite a recent boom in scientific publications and a lot of unfounded claims in the popular press, it is fair to say that the technology readiness level of both 3D and 4D bioprinting is still low. As with any other technology, early “real-life” applications will probably follow the shortest development and regulatory compliance pathway. It can thus be predicted that, besides curiosity-driven knowledge generation and other basic science studies, the development of diagnostic tools or the setup of fairly simple in vitro drug screening tests for the pharmaceutical industry will be the first to reach the market. Use of bioprinted tissues and organs for regenerative medicine presents a few additional technical, logistical, and regulatory challenges, as well as some pending questions associated with cost effectiveness and market access.
Notwithstanding, the lack of suitable bioinks entails a huge interdisciplinary challenge for biochemists and materials scientists, as a multidisciplinary approach is fundamental for the success of the field. Even if synthetic bioinks are constantly being improved, many of the required cell environment features are missing. The embodiment of dECM with the remaining material that consists of ECM fibers, growth factors, and functional and structural proteins will resemble more closely the physiological milieu and could be coupled with the idea of stimuli-responsive bioinks, to better simulate the long pursued in vivo functionality. However, batch-to-batch variations in composition may represent a challenge for naturally sourced dECM-based materials. In conclusion, there is a clear need for the development of not only smart but also monitorable inks that allow live-cell imaging, as well as dynamic imaging techniques that enable tracking of cell-laden 3D printed structures for relatively long time periods.
Acknowledgments
Three-dimensional bioprinting-related work in our laboratories was supported by [A.I.] grants from Instituto de Salud Carlos III (PI19/01621 and PT20/00030), cofunded by ERDF/ESF, “Investing in your future”; Diputación Foral de Gipuzkoa; and the Department of Health (2020111004; 20BU206) and the Department of Economy and Competitiveness of the Basque Government (KK-2020/00010; KK-2019/00006; KK-2019/00093); [L.L.-M.] ERC Advanced Grant “4DbioSERS” #787510 from the European Research Council and Maria de Maeztu Units of Excellence Program from the Ministerio de Ciencia e Innovación through the Spanish State Research Agency (Grant No. MDM-2017-0720). C.G.A. thanks Ministerio de Ciencia e Innovación for a Juan de la Cierva Incorporación Fellowship (IJC2019-040827-I). P.V.-A. was partly supported by a donation made by Asociación Katxalin to Biodonostia in 2019.
Biographies
Paula Vázquez-Aristizabal studied biochemistry and molecular biology at the University of the Basque Country. She holds a MSc in translational medicine from the University of Barcelona. She is a PhD student in the groups of Prof. Ander Izeta (Tissue Engineering, Biodonostia Health Research Institute) and Prof. Luis M. Liz-Marzán (Bionanoplasmonics, CIC biomaGUNE). Her research focuses on the development of nanoparticle-doped bioinks for 3D printing of dynamic cancer models.
Govindaraj Perumal obtained in 2019 a Ph.D. in Biomedical Devices and Technology from the Indian Institute of Technology Madras (IITM), Chennai, India. He worked as postdoctoral research associate at CIC biomaGUNE under the supervision of Prof. Luis M. Liz-Marzán. During his postdoctoral research, he worked on 3D bioprinting of plasmonic nanocomposite scaffolds for biosensing applications. Currently, he is an Associate Professor in the Department of Biomedical Engineering, Rajalakshmi Engineering College, Chennai.
Clara García-Astrain obtained her B.Sc. in chemistry from the University of Navarra (Spain) in 2009. In 2015, she completed her Ph.D. from the University of the Basque Country (Spain) on the development of (bio) polymer-based click cross-linked hydrogels and nanocomposites. From 2016 to 2018, she completed postdoctoral research at the University of Strasbourg (France) and at the Basque Centre for Materials, Applications and Nanostructures (BCMaterials). Currently, she is a Juan de la Cierva postdoctoral fellow at CIC Biomagune in the Bionanoplasmonics lab headed by Prof. Luis M. Liz-Marzán, where she works on the design of polymer-based plasmonic materials for sensing and imaging applications.
Luis Liz-Marzán is Ikerbasque Professor of CIC biomaGUNE, in San Sebastián (Spain), where he served as Scientific Director from 2012 to 2020. He graduated in chemistry from the University of Santiago de Compostela and was a postdoc at Utrecht University and Professor at the University of Vigo (1995–2012), as well as a visiting professor at various research institutions worldwide. Liz-Marzán is considered a pioneer in the colloidal synthesis and self-assembly of metal nanocrystals, as well as the characterization and application of their plasmonic properties. His recent research focuses on the biomedical applications of plasmonic nanostructures.
Ander Izeta obtained his Ph.D. degree in 2000 from the Autonomous University of Madrid, Spain. His current research interests focus on adult stem cell biology, tissue regeneration, and 3D bioprinting.
Author Contributions
A.I. and L.L.-M. conceived the idea of developing the manuscript. P.V.-A. wrote the manuscript and collected most relevant references. All authors contributed in writing and reviewing of the manuscript and approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Lesavage B. L.; Suhar R. A.; Broguiere N.; Lutolf M. P.; Heilshorn S. C. Next-Generation Cancer Organoids. Nat. Mater. 2022, 21, 143–159. 10.1038/s41563-021-01057-5. [DOI] [PubMed] [Google Scholar]
- Su A.; Al’Aref S. J. History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine 2018, 1–10. 10.1016/B978-0-12-803917-5.00001-8. [DOI] [Google Scholar]
- Zandi N.; Sani E. S.; Mostafavi E.; Ibrahim D. M.; Saleh B.; Shokrgozar M. A.; Tamjid E.; Weiss P. S.; Simchi A.; Annabi N. Nanoengineered Shear-Thinning and Bioprintable Hydrogel as a Versatile Platform for Biomedical Applications. Biomaterials 2021, 267, 120476. 10.1016/j.biomaterials.2020.120476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillispie G. J.; Han A.; Uzun-Per M.; Fisher J.; Mikos A. G.; Khan Niazi M. K.; Yoo J. J.; Lee S. J.; Atala A. The Influence of Printing Parameters and Cell Density on Bioink Printing Outcomes. Tissue Eng. Part A 2020, 26 (23–24), 1349–1358. 10.1089/ten.tea.2020.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A.; Levato R.; D’Este M.; Piluso S.; Eglin D.; Malda J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120 (19), 11028–11055. 10.1021/acs.chemrev.0c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasekhar L.; Huynh N. D.; Vecheck A.; Kishore V.; Bashur C. A.; Mitra K. Three-Dimensional Printing of Cell-Laden Microporous Constructs Using Blended Bioinks. J. Biomed. Mater. Res. Part A 2022, 110 (3), 535–546. 10.1002/jbm.a.37303. [DOI] [PubMed] [Google Scholar]
- Crapo P. M.; Gilbert T. W.; Badylak S. F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32 (12), 3233–3243. 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T. W.; Freund J.; Badylak S. F. Quantification of DNA in Biologic Scaffold Materials. J. Surg. Res. 2009, 152 (1), 135–139. 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.; Anil Kumar PR; Yoo J. J.; Zahran F.; Atala A.; Lee S. J. A Photo-Crosslinkable Kidney ECM-Derived Bioink Accelerates Renal Tissue Formation. Adv. Healthc. Mater. 2019, 8 (7), e1800992 10.1002/adhm.201800992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L. P.; Gaspar V. M.; Mendes L.; Duarte I. F.; Mano J. F. Organotypic 3D Decellularized Matrix Tumor Spheroids for High-Throughput Drug Screening. Biomaterials 2021, 275, 120983. 10.1016/j.biomaterials.2021.120983. [DOI] [PubMed] [Google Scholar]
- Chakraborty A.; Roy A.; Ravi S. P.; Paul A. Exploiting the Role of Nanoparticles for Use in Hydrogel-Based Bioprinting Applications: Concept, Design, and Recent Advances. Biomater. Sci. 2021, 9 (19), 6337–6354. 10.1039/D1BM00605C. [DOI] [PubMed] [Google Scholar]
- Zhu K.; Shin S. R.; van Kempen T.; Li Y.-C.; Ponraj V.; Nasajpour A.; Mandla S.; Hu N.; Liu X.; Leijten J.; Lin Y. D.; Hussain M. A.; Zhang Y. S.; Tamayol A.; Khademhosseini A. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27 (12), 1605352. 10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marapureddy S. G.; Hivare P.; Sharma A.; Chakraborty J.; Ghosh S.; Gupta S.; Thareja P. Rheology and Direct Write Printing of Chitosan - Graphene Oxide Nanocomposite Hydrogels for Differentiation of Neuroblastoma Cells. Carbohydr. Polym. 2021, 269, 118254. 10.1016/j.carbpol.2021.118254. [DOI] [PubMed] [Google Scholar]
- Dong L.; Bu Z.; Xiong Y.; Zhang H.; Fang J.; Hu H.; Liu Z.; Li X. Facile Extrusion 3D Printing of Gelatine Methacrylate/Laponite Nanocomposite Hydrogel with High Concentration Nanoclay for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2021, 188, 72–81. 10.1016/j.ijbiomac.2021.07.199. [DOI] [PubMed] [Google Scholar]
- ISO/TC 194. ISO 10993-1:2018 (EN) Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process. https://www.iso.org/obp/ui#iso:std:iso:10993:-1:ed-5:v2:en (accessed 2022-01-18).
- Zhuo X.; Henriksen-Lacey M.; Jimenez de Aberasturi D.; Sanchez-Iglesias A.; Liz-Marzán L. M. Shielded Silver Nanorods for Bioapplications. Chem. Mater. 2020, 32 (13), 5879–5889. 10.1021/acs.chemmater.0c01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez de Aberasturi D.; Henriksen-Lacey M.; Litti L.; Langer J.; Liz-Marzán L. M. Using SERS Tags to Image the Three-Dimensional Structure of Complex Cell Models. Adv. Funct. Mater. 2020, 30 (14), 1909655. 10.1002/adfm.201909655. [DOI] [Google Scholar]
- García-Astrain C.; Lenzi E.; Jimenez de Aberasturi D.; Henriksen-Lacey M.; Binelli M. R.; Liz-Marzán L. M. 3D-Printed Biocompatible Scaffolds with Built-In Nanoplasmonic Sensors. Adv. Funct. Mater. 2020, 30 (45), 2005407. 10.1002/adfm.202005407. [DOI] [Google Scholar]
- Zhang J.; Wehrle E.; Adamek P.; Paul G. R.; Qin X.-H.; Rubert M.; Müller R. Optimization of Mechanical Stiffness and Cell Density of 3D Bioprinted Cell-Laden Scaffolds Improves Extracellular Matrix Mineralization and Cellular Organization for Bone Tissue Engineering. Acta Biomater. 2020, 114, 307–322. 10.1016/j.actbio.2020.07.016. [DOI] [PubMed] [Google Scholar]
- Dogan L.; Scheuring R.; Wagner N.; Ueda Y.; Schmidt S.; Wörsdörfer P.; Groll J.; Ergün S. Human IPSC-Derived Mesodermal Progenitor Cells Preserve Their Vasculogenesis Potential after Extrusion and Form Hierarchically Organized Blood Vessels OPEN ACCESS Human IPSC-Derived Mesodermal Progenitor Cells Preserve Their Vasculogenesis Potential After. Biofabrication 2021, 13 (4), 045028. 10.1088/1758-5090/ac26ac. [DOI] [PubMed] [Google Scholar]
- Feng M.; Hu S.; Qin W.; Tang Y.; Guo R.; Han L. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega 2021, 6 (36), 23067–23075. 10.1021/acsomega.1c02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunti S.; Hoke A. T. K.; Vu K. P.; London N. R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers (Basel). 2021, 13 (4), 874. 10.3390/cancers13040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Wan Z.; Kamm R. D. Vascularized Organoids on a Chip: Strategies for Engineering Organoids with Functional Vasculature. Lab Chip. 2021, 21 (3), 473–488. 10.1039/D0LC01186J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey M.; Ayan B.; Yurieva M.; Unutmaz D.; Ozbolat I. T. Studying Tumor Angiogenesis and Cancer Invasion in a Three-Dimensional Vascularized Breast Cancer. Adv. Biol. 2021, 5 (7), e2100090 10.1002/adbi.202100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardal A.; Zhang J.; McCoard L.; Oottamasathien S.; Prestwich G. D. Dynamically Crosslinked Gold Nanoparticle–Hyaluronan Hydrogels. Adv. Mater. 2010, 22 (42), 4736–4740. 10.1002/adma.201001436. [DOI] [PubMed] [Google Scholar]