Abstract
The ongoing public health emergency of opioid use disorders (OUD) and overdose in the United States is largely driven by fentanyl and its related analogues and has resulted in over 75 673 deaths in 2021. Immunotherapeutics such as vaccines have been investigated as a potential interventional strategy complementary to current pharmacotherapies to reduce the incidence of OUD and opioid-related overdose. Given the importance of targeting structurally distinct fentanyl analogues, this study compared a previously established lead conjugate vaccine (F1–CRM) to a series of novel vaccines incorporating haptens derived from alfentanil and acetylfentanyl (F8, 9a, 9b, 10), and evaluated their efficacy against drug-induced pharmacological effects in rats. While no vaccine tested provided significant protection against alfentanil, lead formulations were effective in reducing antinociception, respiratory depression, and bradycardia elicited by fentanyl, sufentanil, and acetylfentanyl. Compared with control, vaccination with F1–CRM also reduced drug levels in the brain of rats challenged with lethal doses of fentanyl. These data further support investigation of F1–CRM as a candidate vaccine against fentanyl and selected analogues.
Introduction
The epidemic of opioid use disorders (OUD) and drug-related overdose fatalities has impacted the United States for decades1,2 and was exacerbated by the COVID-19 pandemic. Fatal drug overdoses increased by 18% to a total of 92 000 annual deaths by May 2020,3,4 including 62 900 opioid-related deaths. The highly potent synthetic opioid fentanyl and its analogues were implicated in a substantial portion of those fatalities, including incidents involving other opioids5 or nonopioids such as cocaine, methamphetamine, or other substances laced with fentanyl.6,7
Currently available medications, consisting of opioid receptor agonists and antagonists, are effective; but their clinical implementation is limited by side effects, lack of access, stigma associated with opioid agonist therapy, and the requirement for detoxification prior to initiation of antagonist therapy.8,9 Vaccines against fentanyl or other opioids, a proposed alternative or adjunct therapy for OUD, operate through production of target-specific polyclonal antibodies, which sequester drug in the serum and reduce brain exposure to the compound of interest. Such vaccines, which consist of an opioid-based hapten conjugated to an immunogenic carrier protein, have shown substantial preclinical efficacy as a strategy to combat OUD and drug-related overdose (reviewed in refs (10 and 11)). A vaccine targeting oxycodone is currently being investigated in subjects with OUD in Phase I clinical trials.12 Antifentanyl vaccines targeting OUD have shown efficacy in reducing antinociception, respiratory depression, and brain distribution of fentanyl in mice and rats,13−16 and in some studies were effective against potentially lethal fentanyl doses (i.e., 2–4 mg/kg) in mice.16 Because of their selectivity for the target drug, antifentanyl vaccines did not interfere with pharmacological activity of off-target opioids such as methadone and naloxone, or critical care medications such as anesthetics.15 Finally, such vaccines reduced the reinforcing effects of fentanyl in operant behavioral assays.17,18
An important consideration for vaccines targeting the class of fentanyl-like drugs is the prevalence of fentanyl analogues.19 Because vaccine-induced antibodies are highly specific for their target opioid, it is critical for vaccine research to stay ahead of structurally diverse fentanyl analogues.20 In the present study, we explore the utility of fentanyl analogue-derived haptens in conjugate vaccines targeting fentanyl, alfentanil and acetylfentanyl. Alfentanil is a fast-acting fentanyl derivative used for anesthesia,21 and acetylfentanyl (desmethyl fentanyl) is an impurity frequently encountered in illicit fentanyl and is less potent than fentanyl but with a narrow therapeutic window.22 Toxicology results from impaired driving cases show the frequent presence of acetylfentanyl in samples from drivers who tested negative for alcohol, but positive for fentanyl or other substances.23,24 Additionally, acetylfentanyl has been detected in hair samples from people who use heroin,25 and in the urine of patients who tested positive for nonprescribed opioids,26 highlighting the prevalence of fentanyl analogues in illicit mixtures of fentanyl or other opioids.
Previous reports of antiopioid vaccines have shown some ability of antifentanyl vaccines to generate cross-protective antibodies against fentanyl analogues. Other groups have demonstrated in vitro binding of vaccine-induced polyclonal antibodies to structurally related fentanyl analogues, such as acetylfentanyl and α-methylfentanyl; however, most of these studies did not evaluate the efficacy of such antibodies against these analogues in vivo.14,16,27−29 Our previous studies of conjugate vaccines utilizing a series of fentanyl-based haptens (F1–F6) conjugated to either diphtheria toxoid cross reactive material-197 (CRM, or CRM197) or keyhole limpet hemocyanin (KLH) carrier proteins showed promising efficacy in rats against fentanyl, limited protection against sufentanil, and no in vivo efficacy or in vitro cross-reactivity against alfentanil,13,15 supporting the exploration of alternate fentanyl-based haptens to achieve efficacy against fentanyl analogues. An additional carfentanil hapten (F7) was evaluated but discarded from further advancement (data not shown). Here, we compared the efficacy of a previously reported fentanyl vaccine (F1–CRM) to novel conjugate vaccines designed to target alfentanil (F8–CRM), fentanyl (F9a–CRM and F9b–CRM), and acetylfentanyl (F10–CRM). Vaccines were evaluated for in vivo efficacy against drug-induced antinociception, respiratory depression, and bradycardia, while in vitro binding of polyclonal IgG antibodies to fentanyl and fentanyl analogues was characterized by ELISA and biolayer interferometry (BLI). Finally, the lead vaccine F1–CRM was evaluated for its efficacy against a lethal dose of fentanyl in rats. These results can be used to assess interaction between hapten structure, polyclonal antibody affinity, and in vivo efficacy of vaccines against fentanyl analogues to inform the design of vaccines targeting multiple fentanyl-class compounds.
Results
Synthesis of Conjugate Vaccines against Fentanyl and Fentanyl Analogues
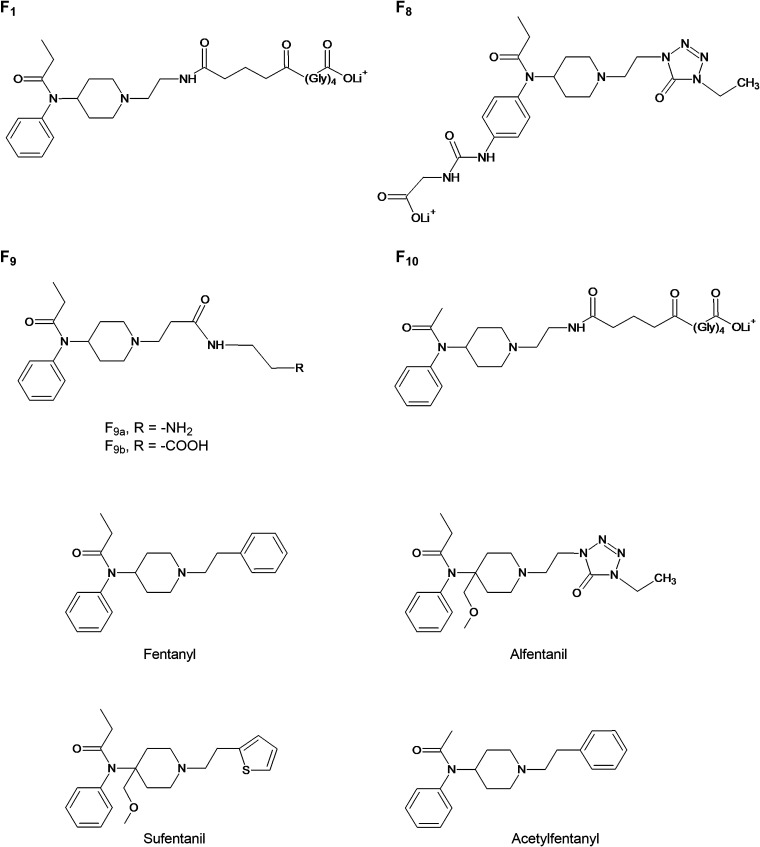
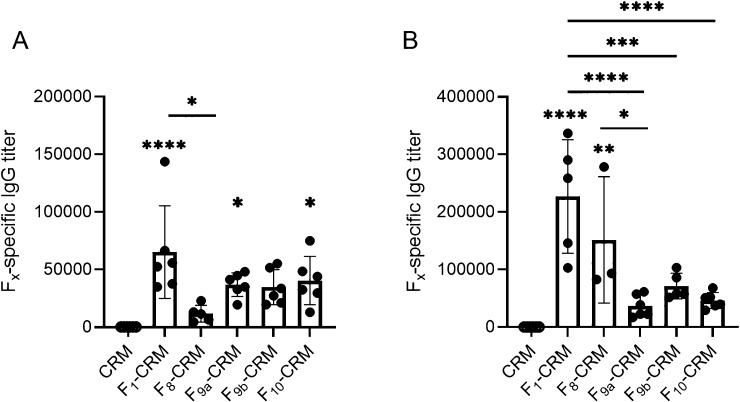
Haptens (Figure 1) were synthesized on the basis of the structures of alfentanil (F8), fentanyl (F9a, F9b), and acetylfentanyl (F10). Hapten structures were confirmed by 1H NMR (see Supporting Information), and haptens were conjugated to CRM carrier protein. Conjugates were characterized by MALDI-TOF to determine the haptenation ratio (Table SI, Supporting Information). Because conjugate vaccines incorporating the F1 hapten have previously shown efficacy against fentanyl,13,15 a lead F1–CRM vaccine was included to provide a basis of comparison for vaccine efficacy. Rats were immunized with CRM control; F1–CRM; or novel conjugates F8–CRM, F9a–CRM, F9b–CRM, or F10–CRM adjuvanted with aluminum hydroxide (alum, Alhydrogel-85). Hapten-specific polyclonal serum IgG antibodies elicited by the vaccine were evaluated by ELISA on day 49 (Figure 2A), and at the termination of the experiment (Figure 2B). All vaccines elicited detectable titers against their cognate hapten.
Figure 1.
Structures of haptens targeting fentanyl and its analogues. A series of novel haptens based on the structures of fentanyl (F1, F9a, F9b), alfentanil (F8), and acetylfentanyl (F10). The F1 hapten has been previously described.
Figure 2.
Vaccination with conjugates containing F8–10 haptens elicits hapten-specific IgG titers. Sprague–Dawley rats (n = 6 per group) were given an intramuscular (i.m.) immunization on days 0, 21, 42, and 63 with conjugate vaccines containing the F1 or F8–10 haptens conjugated to CRM. Hapten-specific serum IgG antibody titers were evaluated by ELISA (A) 1 week after the third immunization (day 49), and (B) after completion of drug challenges (day 105). Data are expressed as mean ± SEM. Symbols: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 vs CRM control. Brackets indicate pairwise group comparisons.
Efficacy of Conjugate Vaccines against Fentanyl and Fentanyl Analogues in Rats
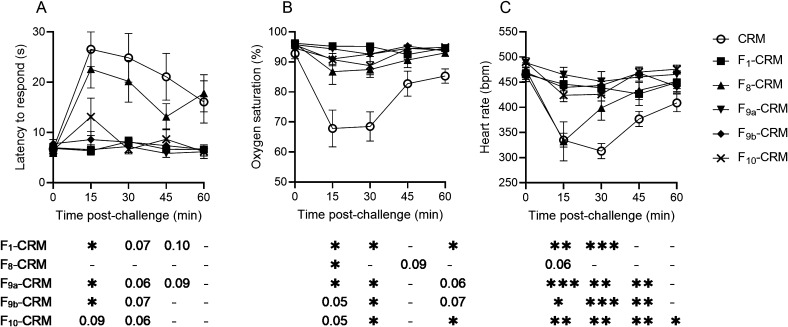
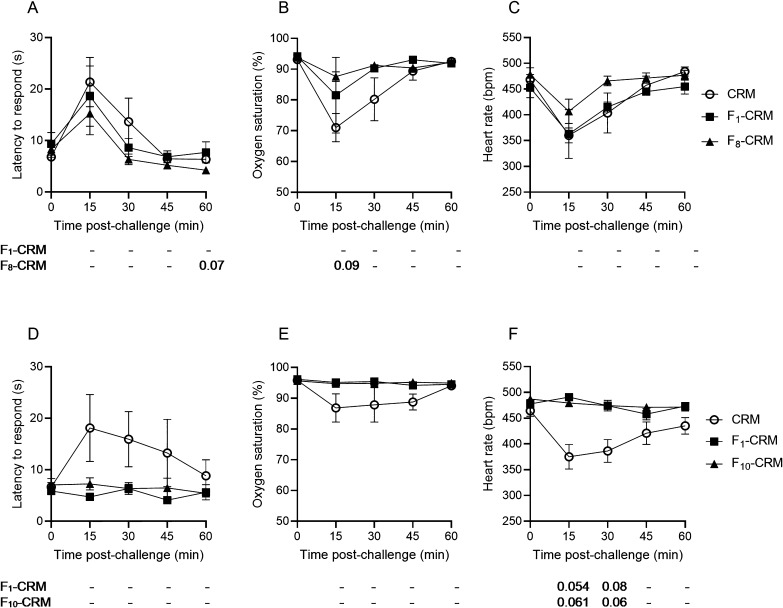
Following the third vaccination, rats were challenged with fentanyl, alfentanil, and acetylfentanyl, with a minimum washout period of 1 week between challenges. In order to control for tolerance effects, rats were randomized into one of three challenge groups, in which the order of drug challenges was rotated (such that 2 rats per group received fentanyl, alfentanil or acetylfentanyl in each challenge). The F1–CRM, F9a/9b–CRM, and F10–CRM conjugate vaccines were protective against the effects of fentanyl (Figure 3), and rats vaccinated with F8–CRM showed some degree of protection, though only the effect on oxygen saturation was significant (Figure 3B). However, none of the vaccine groups showed any protection against alfentanil compared to CRM control (Figure S1A–C), and only F9a/9b–CRM and F10–CRM were protective against acetylfentanyl-induced bradycardia, but not against antinociception or respiratory depression (Figure S1D–F).
Figure 3.
Efficacy of the vaccines containing the F8–10 haptens against fentanyl. Sprague–Dawley rats (n = 6, each group) were given an intramuscular (i.m.) vaccination on days 0, 21, 42, and 63 with conjugate vaccines containing the F1 and F8–10 haptens or with CRM control, and were then challenged with 0.1 mg/kg fentanyl, subcutaneous (s.c.). Rats were monitored at 15 min intervals for (A) antinociception by latency to respond on a hot plate and for (B) oxygen saturation (%) and (C) heart rate measured by pulse oximetry. Data are expressed as mean ± SEM. Below their respective graphical panels, significance of each vaccine group vs CRM control is indicated at each time point. Symbols: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to control; exact p-values are listed for 0.05 ≤ p ≤ 0.1; and “–” indicates no significant difference, 0.10 ≤ p.
Efficacy of F8–CRM against Alfentanil and F10–CRM against Acetylfentanyl
Because the dose of these analogues in the first set of challenges (0.5 mg/kg alfentanil and 0.5 mg/kg acetylfentanyl) showed a strong effect from alfentanil but only a mild effect from acetylfentanyl by antinociception and respiratory depression in CRM control rats, we hypothesized that a lower dose of alfentanil and a higher dose of acetylfentanyl may have been required to determine whether the vaccine was able to protect from these drugs. That is, a dose of 0.5 mg/kg alfentanil may be high enough to overcome the ability of vaccine-elicited antibodies to sequester the drug, whereas 0.5 mg/kg acetylfentanyl was insufficient to produce robust antinociception, respiratory depression, or bradycardia.
To further evaluate the efficacy of vaccines against alfentanil and acetylfentanyl, rats were separated into two groups and challenged with either a lower dose of alfentanil, 0.25 mg/kg, or a higher dose of acetylfentanyl, 1.0 mg/kg (Figure 4). Rats vaccinated with F8–CRM were challenged with alfentanil, rats vaccinated with F10–CRM were challenged with acetylfentanyl, and rats vaccinated with CRM control or F1–CRM were divided equally between the two challenge treatment groups. In this scenario, F8–CRM and F1–CRM did not provide significant protection against alfentanil; and the effect of F1–CRM and F10–CRM on acetylfentanyl-induced pharmacological effects was not statistically significant compared to CRM, likely due to the small sample size of the divided CRM control and F1–CRM groups (n = 3 per group).
Figure 4.
Efficacy of the vaccines containing the F8 and F10 haptens against alfentanil and acetylfentanyl, respectively. Rats immunized with control, F1–CRM, or F8–CRM were challenged with 0.25 mg/kg alfentanil, subcutaneous (s.c.) (A–C); and rats immunized with control, F1–CRM, or F10–CRM were challenged with 1.0 mg/kg acetylfentanyl s.c. (D–F). Rats were monitored at 15 min intervals for: (A,D) antinociception by latency to respond on a hot plate; (B,E) oxygen saturation (%); and (C,F) heart rate measured by pulse oximetry. Data are expressed as mean ± SEM. Below the graphs, significance of each vaccine group vs CRM control is indicated compared to control at each time point. Symbols and statistics: exact p-values are listed for 0.05 ≤ p ≤ 0.1, and “–” indicates no significant difference, 0.10 ≤ p.
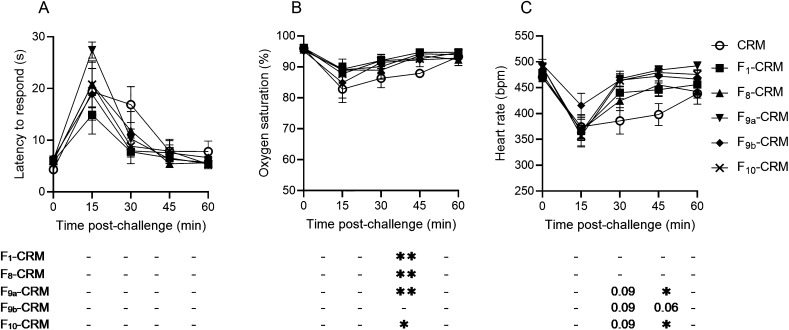
Efficacy of Conjugate Vaccines against Sufentanil
After completion of fentanyl, alfentanil, and acetylfentanyl challenges, rats were given one additional vaccination on day 84. Two weeks after the boost, on day 98, rats were challenged a final time with sufentanil, 0.008 mg/kg (Figure 5). None of the vaccinated rats showed significant protection from the antinociceptive effects of sufentanil; however, F1–CRM, F8–CRM, F9a–CRM, and F10–CRM vaccinated groups showed increased oxygen saturation at later time points (Figure 5B), indicating more rapid recovery from the effects of sufentanil.
Figure 5.
Efficacy of vaccines containing F8–10 haptens against sufentanil. Rats vaccinated with F1 and F8–10 conjugate vaccines or CRM control were challenged with 0.008 mg/kg sufentanil, subcutaneous (s.c.). Rats were monitored at 15 min intervals for (A) antinociception by latency to respond on a hot plate, B) oxygen saturation (%), and (C) heart rate. Data are expressed as mean ± SEM. Below their respective graphs, significance of each vaccine group vs CRM control is indicated at each time point. Symbols and statistics: *p ≤ 0.05, **p ≤ 0.01 compared to control; p-values are listed for 0.05 ≤ p ≤ 0.1; and “–” indicates no significant difference, 0.10 ≤ p.
Relative Affinity of Serum Antibodies for Fentanyl and Fentanyl Analogues
After a washout period of one week following the final challenge, blood was collected for analysis of serum antibody level and polyclonal antibody relative affinity by competitive ELISA (Table 1). Sera from all vaccine groups showed nanomolar IC50 values for fentanyl, though sera from the F1–CRM group showed the lowest IC50. None of the sera showed significant binding to alfentanil, with all showing IC50 values above 100 μM. Similarly, affinity of all sera for sufentanil was in the micromolar range, with F1–CRM, F9a–CRM, and F10–CRM serum showing IC50 values between 20 and 30 μM. F10–CRM, the hapten with the most structural similarity to acetylfentanyl, produced antibodies with the greatest affinity for acetylfentanyl, with IC50 of 6.99 nM, though F1–CRM serum also showed a relatively high affinity with an IC50 of 28.6 nM.
Table 1. F8–10 Relative Affinitya.
| F1–CRM | F8–CRM | F9a–CRM | F9b–CRM | F10–CRM | |
|---|---|---|---|---|---|
| fentanyl (nM) | 17.2 ± 14.8 | 141.3 ± 63.5 | 25.9 ± 17.7 | 69.7 ± 69.2 | 50.1 ± 26.1 |
| alfentanil (μM) | 243 | 348 | 203.9 | 181.4 | 180.2 |
| acetylfentanyl (nM) | 28.59 | 2090 | 584 | 763 | 6.99 |
| sufentanil (μM) | 25.82 | >100 | 20.81 | >100 | 29.04 |
Fentanyl IC50 expressed as mean ± SEM from all sera in group, n = 6; IC50 for analogues obtained using serum pooled from all samples in each group. Maximum detection limit for alfentanil was 1 mM; detection limit for fentanyl, sufentanil and acetylfentanyl was 100 μM.
In order to estimate cross-reactivity between polyclonal antibodies against fentanyl-, alfentanil-, and acetylfentanyl-targeting haptens, in vitro binding of pooled serum from each vaccine group was evaluated against biotinylated F1, F8, and F10 haptens by biolayer interferometry (BLI). Serum from all vaccine groups showed binding to both F1-biotin and F10-biotin, and only serum from F8–CRM vaccinated rats showed significant interaction with F8-biotin (Figure S2). Serum from rats vaccinated with F1–CRM showed the highest response values to both F1-biotin and F10-biotin, consistent with the F1-specific titer obtained by ELISA (Figure 2).
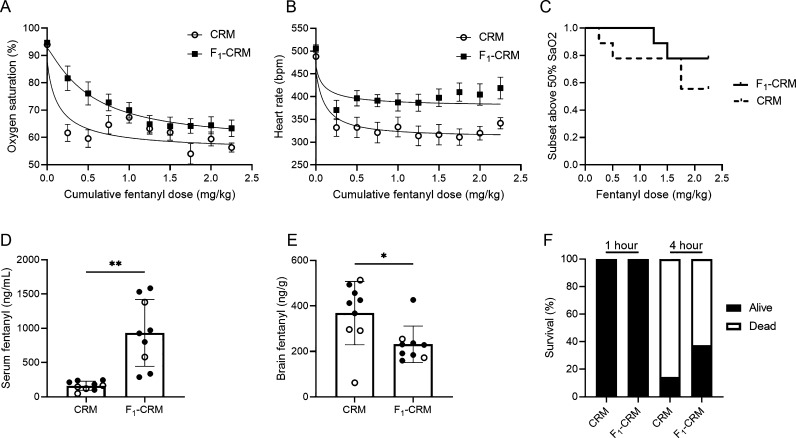
Efficacy of Lead Conjugate Vaccine F1–CRM against High Fentanyl Doses
To evaluate efficacy of the lead F1–CRM vaccine against high doses of fentanyl, a separate cohort of rats was vaccinated with F1–CRM or with CRM control. After the last vaccination, rats were given doses of 0.25 mg/kg of fentanyl every 15 min to a total cumulative dose of 2.25 mg/kg (Figure 6). The F1–CRM vaccine shifted the ED50 for respiratory depression approximately 3.5-fold from 0.139 to 0.523 mg/kg (Figure 6A), and reduced fentanyl-induced bradycardia (Figure 6B). Individual rats were euthanized if oxygen saturation dropped below 50% (Figure 6C), or after the final fentanyl dose was administered, and brain and serum were collected for analysis of fentanyl distribution. The overall effect of F1–CRM on preventing respiratory arrest was not significant (p = 0.35), though F1–CRM-immunized rats showed increased concentration of fentanyl in serum and reduced distribution to brain (Figure 6D,E). To determine the effect of F1–CRM vaccine on mortality from acute fentanyl exposure, a separate cohort of rats was vaccinated with F1–CRM or with CRM control. After the last vaccination, rats were given a bolus dose of 2.25 mg/kg, and survival was recorded at 1 and 4 h postfentanyl challenge (Figure 6F). At 4 h postadministration more rats survived in the vaccinated group compared to control, though the difference was not significant.
Figure 6.
Efficacy of a fentanyl vaccine against higher fentanyl doses in rats. Rats (n = 9 per group) were given an intramuscular (i.m.) immunization on days 0, 21, 42, and 63 with either CRM control or the F1–CRM conjugate adsorbed on alum. A week after the fourth vaccination, rats were challenged with doses of 0.25 mg/kg fentanyl subcutaneous (s.c.) every 15 min, to a final cumulative dose of 2.25 mg/kg or until oxygen saturation was measured at <50%. (A) Respiratory depression measured by oxygen saturation (%) and (B) bradycardia measured as heart rate (bpm) over the course of the experiment. (C) Survival curve indicating subset of rats above 50% oxygen saturation; once <50% oxygen saturation was reached or at a cumulative dose of 2.25 mg/kg fentanyl, rats were euthanized and fentanyl concentration was quantified in serum and brain tissue. Fentanyl concentration calculated in (D) serum and (E) brain; open circles indicate rats that did not receive the full cumulative dose. Data are expressed as mean ± SEM. Symbols: *p ≤ 0.05; **p ≤ 0.01. (F) A separate cohort of rats was immunized as above and challenged with a bolus dose of 2.25 mg/kg fentanyl. Survival was assessed at 1 and 4 h postfentanyl.
Discussion
This study sought to evaluate the efficacy of novel antialfentanil and antiacetylfentanyl conjugate vaccines against fentanyl and selected fentanyl analogues in rats. The efficacy of these and of the antifentanyl vaccines F1–CRM and F9a/9b–CRM against fentanyl analogues including alfentanil, sufentanil, and acetylfentanyl, were fully characterized by evaluating the relative affinity of antibodies against these analogues in vitro, and in vivo efficacy was tested against opioid-induced antinociception, respiratory depression, and bradycardia. This approach builds upon reports of other antifentanyl and antifentanyl analogue vaccines currently under investigation. Specifically, other groups have demonstrated in vitro affinity of fentanyl vaccine-induced polyclonal antibodies for some structurally related fentanyl analogues, such as acetylfentanyl and α-methylfentanyl, but the efficacy of such antibodies against these compounds was not evaluated in vivo.14,16 Evaluations of multitarget vaccines such as heroin/fentanyl vaccines have shown specificity of serum antibodies for multiple opioid targets but did not assess efficacy against fentanyl analogues other than those targeted by the vaccine.27,28,30−32 Recent vaccines targeting carfentanil have demonstrated in vitro cross-reactivity against multiple fentanyl analogues and in vivo efficacy against both fentanyl and carfentanil,29 and against fentanyl–carfentanil admixtures,100 but were not evaluated in vivo against other fentanyl analogues.
In the present study, all vaccines generated fentanyl-specific antibody titers and were protective against 0.1 mg/kg fentanyl to some degree, though the antialfentanil F8–CRM was least efficacious. This result matched with the in vitro affinity of antibodies for fentanyl measured by competitive ELISA, in which F8–CRM serum showed the lowest relative affinity for fentanyl in vitro (Table 1). In initial challenges of 0.5 mg/kg alfentanil and acetylfentanyl, none of the vaccines were protective against alfentanil, and only F9a–CRM, F9b–CRM, and F10–CRM were effective at reducing acetylfentanyl-induced bradycardia. In follow-up experiments using 0.25 mg/kg alfentanil, F8–CRM did not have significant impact on alfentanil-induced effects; and with 1.0 mg/kg acetylfentanyl, the effects of F1–CRM and F10–CRM were not statistically significant (p = 0.08 and p = 0.06, respectively); however, it is possible that F10–CRM would be protective in a challenge with higher statistical power (n > 6). Finally, some degree of efficacy was seen against 0.008 mg/kg sufentanil, which is consistent with previous reports of F1–CRM vaccine efficacy against this fentanyl analogue in rats.15
Differences in efficacy can be attributed in part to conjugate properties including hapten chemistry, linker length, and haptenation ratio (Table SI, Supporting Information). The F10 hapten differs from F1 only in the presence of the methyl group that distinguishes fentanyl from acetylfentanyl, and both conjugates displayed similar haptenation ratios. The F1–CRM and F10–CRM vaccines produced serum antibody response with nanomolar affinity for both fentanyl and acetylfentanyl (Table 1), with F1–CRM producing higher relative affinity for fentanyl (17.2 nM) and F10–CRM producing higher relative affinity for acetylfentanyl (6.99 nM). In comparison to F1–CRM, F9a–CRM and F9b–CRM had shorter linker length and lower haptenation ratios (17.0 for F1–CRM vs 4.2 for F9a–CRM and 8.5 for F9b–CRM), and though all three conjugates produced antibodies with nanomolar affinity for fentanyl, F1–CRM generated antibodies showing the lowest IC50 (Table 1).
Interestingly, F8–CRM was effective against fentanyl in vivo but not against alfentanil. The relative affinity of serum from rats vaccinated with F8–CRM indicated substantially higher affinity of polyclonal antibodies for fentanyl than for alfentanil (141 nM vs 348 μM). Conversely, BLI analysis of F8–CRM binding to the biotinylated haptens indicated relatively similar levels of binding of serum antibodies to F1, F8, and F10. Importantly, the F8 hapten differs from the alfentanil molecule in that it lacks the methoxymethyl moiety on the 4-position of the central piperidine, possibly accounting for the low relative affinity of F8-immunized serum for free alfentanil observed in the competitive ELISA assay.
Differences in efficacy may also arise from the relative potencies of fentanyl versus its analogues; as previously reported,15 testing of vaccine efficacy across multiple analogues in vivo is complicated by different pharmacokinetic and pharmacodynamic profiles of the individual target drugs. For example, in this study alfentanil and acetylfentanyl required higher doses to achieve a clinically relevant effect compared with fentanyl (Figure 4, Figure S2). Whereas a dose of 0.1 mg/kg fentanyl induced an approximately 30% reduction in oxygen saturation in CRM control-vaccinated rats (Figure 3), a 2.5- to 5-fold higher dose of alfentanil was required to produce the same effect. Conversely, sufentanil required a lower dose (0.008 mg/kg) to produce a reduction in oxygen saturation (Figure 5). Therefore, it is possible that even a small amount of cross-reactive antibodies may be sufficient to reduce brain concentration of sufentanil to a measurable effect from vaccine. Hence, it is important to evaluate vaccine efficacy against a battery of opioid-induced pharmacological effects.
Because of its demonstrated efficacy in previous work and here, F1–CRM was concluded to be the best candidate for further preclinical development out of the conjugates evaluated. The lead vaccine F1–CRM produced antibodies with the highest in vitro affinity for fentanyl and was effective at preventing fentanyl-induced respiratory depression and bradycardia. Therefore, it was selected for additional efficacy testing against higher fentanyl doses. This is one of few reports specifically testing antifentanyl vaccines against a lethal fentanyl challenge in rodents, though the overall effect of F1–CRM on survival was not significant.16 Given that control and actively vaccinated rats were resilient to fentanyl-induced fatal overdose particularly in the first 1–2 h after exposure, it is possible that rodents are not suitable species to test for the efficacy of medications against opioid-induced overdose because very high doses of opioids are required for lethality, which may translate into higher drug plasma concentrations than those found in humans. It is indeed possible that other species, for example, ferrets33 or large animals (e.g., pigs34,35) may be required to study clinically relevant overdose scenarios. Overall, these results highlight the importance of evaluating the applicability of antifentanyl vaccines in vivo both for broad efficacy against a variety of fentanyl analogues of clinical interest, and for efficacy of fentanyl vaccines against high doses of fentanyl relevant for use as a strategy for overdose prevention.
Experimental Procedures
Synthesis and Conjugation of F8–10-Based Conjugate Vaccines
See the Supporting Information for details of hapten synthesis and conjugation. Haptens were synthesized and then conjugated by carbodiimide coupling chemistry to CRM from either Fina Biosolutions (F9a, F9b, F10) or Pfenex (F1, F8) for vaccines, or to bovine serum albumin (BSA) for ELISA. The F1–CRM vaccine was prepared as previously described.15 Conjugates were purified by ultrafiltration (Amicon) and stored at 2.5 mg/mL in sterile PBS pH 7.2.
Animals
All studies were approved by the University of Minnesota Institutional Animal Care and Use Committee, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th ed. Male Sprague–Dawley rats (Envigo), 8 weeks on arrival, were housed with a 14/10 h light/dark cycle and fed ad libitum. Rats were allowed 1 week habituation period prior to initiation of experiments.
Immunization
Vaccines consisted of 60 μg CRM control or F1–CRM or F8–10–CRM conjugate adsorbed on 90 μg alum adjuvant (Alhydrogel-85, Invivogen) in sterile saline to a final volume of 150 μL. Rats (n = 6 per group) were given an intramuscular (i.m.) immunization in both rear thigh muscles (75 μL each side) on days 0, 21, 42, 63, and 84. Serum samples were collected for analysis of serum antibody level by tail vein sampling on day 49, 1 week after the third vaccination, and on day 105, 1 week after completion of all drug challenges.
Analysis of Serum Antibody Level
Antibody analysis was performed via indirect ELISA; briefly, 96-well plates were coated with 5 ng/well of the corresponding BSA conjugate or unconjugated BSA as control in 50 mM Na2CO3 buffer, pH 9.6 (Sigma-Alrdich, St. Louis, MO), and blocked with 1% porcine gelatin (Sigma-Aldrich). Plates were incubated with serum samples diluted in 1× PBS + 0.05% Tween-20 (PBS-T, Thermo Fisher), then washed and incubated with a horseradish peroxidase (HRP)-conjugated goat antirat IgG (Jackson ImmunoResearch) to assess hapten-specific serum IgG. HRP activity was quantitated with o-phenylenediamine substrate (SigmaFast OPD, Sigma-Aldrich) by absorbance at 492 nm on a 96-well plate reader (Tecan Infinite).
Competitive Binding ELISA
Determination of relative affinity by competitive binding ELISA was performed essentially as described.15 Briefly, 96-well plates were coated with 0.5 ng/well F3–BSA36 and blocked with 1% gelatin, and fentanyl or fentanyl analogues were added to the wells with concentrations ranging from 1 × 10–4 M to 1 × 10–10 M. Plates were incubated with diluted serum in the presence of a competitor, washed with PBS-T, incubated with HRP-conjugated goat antirat IgG, and quantitated with OPD substrate as above. The relative affinity of serum antibodies was calculated as IC50, or the concentration of competitor that resulted in 50% decrease in antibody binding.
Drug Challenges
Fentanyl citrate, alfentanil HCl, and sufentanil citrate were obtained from Boynton Pharmacy (University of Minnesota). Acetylfentanyl HCl was obtained through the NIDA drug supply. For acute drug challenges, each drug was diluted in sterile saline and given as a single bolus dose administered subcutaneous (s.c.). Challenges occurred across 3 weeks starting on day 56, with each subject receiving one drug challenge per week. Rats were randomized to receive fentanyl (0.1 mg/kg), alfentanil (0.5 mg/kg), or acetylfentanyl (0.5 mg/kg) in the first challenge, followed by the other analogues in subsequent challenges, in order to control for tolerance effects due to repeated exposure to fentanyl and fentanyl analogues. On day 77, after the initial three challenges were completed, alfentanil (0.25 mg/kg) and acetylfentanyl (1.0 mg/kg) were given to the corresponding vaccine group (F8–CRM for alfentanil or F10–CRM for acetylfentanyl), with CRM control and F1–CRM groups randomized to receive either alfentanil or acetylfentanyl. A final challenge of sufentanil (0.008 mg/kg) was given to all groups on day 98.
Opioid-Induced Antinociception, Respiratory Depression, and Bradycardia
Rats were allowed to acclimate to the testing environment for 1 h prior to experiments, and baseline measurements were taken 15 min prior to drug challenge. Opioid-induced antinociception was measured by latency to respond on a hot plate (Columbus Instruments, Columbus, OH) set to 54 °C, and opioid-induced respiratory depression (percent oxygen saturation, SaO2) and bradycardia (heart rate in beats per minute, BPM) were measured with a MouseOx Plus pulse oximeter (Starr Life Sciences, Oakmont, PA). Antinociception and oximetry measurements were taken at 15 min intervals postdrug administration for a total of 60 min.
F1–CRM Vaccine Efficacy in Lethal Fentanyl Challenge
Rats (n = 9 per group) were given an intramuscular (i.m.) vaccination on days 0, 21, 42, and 70 with either F1–CRM or CRM control. Serum was collected via tail vein sampling on day 50 for analysis of fentanyl-specific antibody levels. On day 84, rats were allowed to acclimate to the testing environment for 1 h, followed by a baseline measurement of oxygen saturation and heart rate. Rats were then given 0.25 mg/kg fentanyl s.c. every 15 min, to a maximum cumulative dose of 2.25 mg/kg. Prior to each successive dose, rats were monitored by oximetry for respiratory depression and bradycardia. Following the final oximetry measurement, or after occurrence of respiratory arrest, rats were euthanized via CO2 inhalation, and blood and brain were collected for analysis of fentanyl concentration by liquid chromatography coupled with mass spectrometry (LC–MS).
LC–MS Analysis of Fentanyl Concentration
Determination of fentanyl concentration in brain and serum was performed as described.13,15 Briefly, serum was prepared from whole blood by centrifugation, and brain tissue was homogenized; serum and brain homogenate were processed with acetonitrile, supernatant was extracted with Bond Elut extraction cartridges (Agilent), and the serum and brain were reconstituted in ammonium formate mobile phase buffer. Samples were analyzed on reverse-phase C18 column coupled with G6470 triple quadrupole mass spectrometry system (Agilent), and peak integration was performed with Mass Hunter software.
Statistical Analysis
Fentanyl-specific serum IgG antibody titers were compared by one-way ANOVA followed by Sidak’s multiple comparisons test. Latency to respond on hot plate, oxygen saturation (SaO2), and heart rate (beats per minute, BPM) over time were compared using two-way ANOVA or mixed-effects analysis with Dunnett’s multiple comparisons test to evaluate significance versus the CRM control group at each time point. Fentanyl-induced mortality during lethal fentanyl overdose was analyzed with Fisher’s exact test. Fentanyl serum and brain concentrations were compared with Welch’s t test. All analyses were conducted in Prism v9.1 (GraphPad, San Diego, CA).
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIDA) and the National Institute of Neurological Disorders and Stroke (NINDS) under grant UG3-DA048386 (M.P.).
Glossary
Abbreviations
- OUD
opioid use disorder
- CRM
diphtheria toxoid cross-reactive molecule
- BSA
bovine serum albumin
- KLH
keyhole limpet hemocyanin
- BLI
biolayer interferometry
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00820.
Details on hapten synthesis and conjugation, conjugate characterization, assessment of serum by BLI, in vivo data from additional alfentanil and acetylfentanyl challenges, and tables comparing haptenation ratio of conjugates and overall efficacy of conjugates against described drug challenges (PDF)
Author Contributions
S.E.A., S.P.R., and M.P. conceived of the project and designed the experiments. C.B., C.R., A.K., R.J., and V.G. performed the experiments. C.B. and M.P. wrote and edited the manuscript.
The authors declare the following competing financial interest(s): Pravetoni, Averick, and Runyon are inventors of provisional application No. 62/989,41, "Fentanyl haptens, fentanyl hapten conjugates, and methods for making and using." The other authors declare no competing financial interest.
Supplementary Material
References
- Wilson N.; Kariisa M.; Seth P.; Smith H.; Davis N. L. Drug and Opioid-Involved Overdose Deaths - United States, 2017–2018. Morb. Mortal. Wkly. Rep. 2020, 69 (11), 290–297. 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Wide-ranging OnLine Data for Epidemiologic Research (WONDER). http://wonder.cdc.gov.
- Alter A. Y. C.COVID-19 Impact on US National Overdose Crisis. Overdose Detection Mapping Application Program, 2020.
- Kosten T. R.; Petrakis I. L. The Hidden Epidemic of Opioid Overdoses During the Coronavirus Disease 2019 Pandemic. JAMA Psychiatry 2021, 78 (6), 585–586. 10.1001/jamapsychiatry.2020.4148. [DOI] [PubMed] [Google Scholar]
- Sutter M. E.; Gerona R. R.; Davis M. T.; Roche B. M.; Colby D. K.; Chenoweth J. A.; Adams A. J.; Owen K. P.; Ford J. B.; Black H. B.; Albertson T. E. Fatal Fentanyl: One Pill Can Kill. Acad. Emerg. Med. 2017, 24 (1), 106–113. 10.1111/acem.13034. [DOI] [PubMed] [Google Scholar]
- Fairbairn N.; Coffin P. O.; Walley A. Naloxone For Heroin, Prescription Opioid, And Illicitly Made Fentanyl Overdoses: Challenges And Innovations Responding To A Dynamic Epidemic. Int. J. Drug Policy 2017, 46, 172–179. 10.1016/j.drugpo.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K.; Milloy M.-J.; Lysyshyn M.; DeBeck K.; Nosova E.; Wood E.; Kerr T. Substance Use Patterns Associated with Recent Exposure to fentanyl among People Who Inject Drugs in Vancouver, Canada: A Cross-Sectional Urine Screening Study. Drug Alcohol Depend. 2018, 183, 1–6. 10.1016/j.drugalcdep.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl J.; Zimmerman D.; Bridgeman P. Medications for Management of Opioid Use Disorder. Am. J. Health-Sys. Pharm. 2019, 76 (15), 1097–1103. 10.1093/ajhp/zxz105. [DOI] [PubMed] [Google Scholar]
- Bell J.; Strang J. Medication Treatment of Opioid Use Disorder. Biol. Psychiatry 2020, 87 (1), 82–88. 10.1016/j.biopsych.2019.06.020. [DOI] [PubMed] [Google Scholar]
- Pravetoni M.; Comer S. D. Development of Vaccines to Treat Opioid Use Disorders and Reduce Incidence of Overdose. Neuropharmacology 2019, 158, 107662. 10.1016/j.neuropharm.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend E. A.; Banks M. L. Preclinical Evaluation of Vaccines to Treat Opioid Use Disorders: How Close Are We to a Clinically Viable Therapeutic?. CNS Drugs 2020, 34 (5), 449–461. 10.1007/s40263-020-00722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh M. D.; King S. J.; Baruffaldi F.; Saykao A.; Hamid F. A.; Winston S.; LeSage M. G.; Pentel P. R.; Pravetoni M. Pharmacological Mechanisms Underlying the Efficacy of Antibodies Generated by a Vaccine to Treat Oxycodone Use Disorder. Neuropharmacology 2021, 195, 108653. 10.1016/j.neuropharm.2021.108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh M. D.; Baruffaldi F.; Peterson S. J.; Le Naour M.; Harmon T. M.; Vigliaturo J. R.; Pentel P. R.; Pravetoni M. A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 2019, 368 (2), 282–291. 10.1124/jpet.118.253674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R. C.; Bow E. W.; Whalen C.; Torres O. B.; Sulima A.; Beck Z.; Jacobson A. E.; Rice K. C.; Matyas G. R. Novel Vaccine That Blunts Fentanyl Effects and Sequesters Ultrapotent Fentanyl Analogues. Mol. Pharmaceutics 2020, 17 (9), 3447–3460. 10.1021/acs.molpharmaceut.0c00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C.; Gradinati V.; Hamid F.; Baehr C.; Crouse B.; Averick S.; Kovaliov M.; Harris D.; Runyon S.; Baruffaldi F.; LeSage M.; Comer S.; Pravetoni M. Therapeutic and Prophylactic Vaccines to Counteract Fentanyl Use Disorders and Toxicity. J. Med. Chem. 2020, 63 (23), 14647–14667. 10.1021/acs.jmedchem.0c01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer P. T.; Kimishima A.; Schlosburg J. E.; Zhou B.; Collins K. C.; Janda K. D. Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem. 2016, 128 (11), 3836–3839. 10.1002/ange.201511654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend E. A.; Blake S.; Faunce K. E.; Hwang C. S.; Natori Y.; Zhou B.; Bremer P. T.; Janda K. D.; Banks M. L. Conjugate Vaccine Produces Long-Lasting Attenuation of Fentanyl vs. Food Choice and Blocks Expression of Opioid Withdrawal-Induced Increases in Fentanyl Choice in Rats. Neuropsychopharmacology 2019, 44 (10), 1681–1689. 10.1038/s41386-019-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney R. D.; Blake S.; Bremer P. T.; Zhou B.; Hwang C. S.; Poklis J. L.; Janda K. D.; Banks M. L. Vaccine Blunts Fentanyl Potency in Male Rhesus Monkeys. Neuropharmacology 2019, 158, 107730. 10.1016/j.neuropharm.2019.107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian P.; Vo K. T.; Barr-Walker J.; Lynch K. L. Fentanyl, Fentanyl Analogs and Novel Synthetic Opioids: A Comprehensive Review. Neuropharmacology 2018, 134 (Part A), 121–132. 10.1016/j.neuropharm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Tunstall B. J.; Vendruscolo L. F. Utility of Fentanyl Vaccines: Unique Challenges Posed by Preventing Opioid Overdose and Treating Opioid Use Disorder. Neuropsychopharmacology 2019, 44 (10), 1675–1676. 10.1038/s41386-019-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde M.; Pichini S.; Pacifici R.; Tagliabracci A.; Busardò F. P.; Auwärter V.; Solimini R. Metabolic Pathways and Potencies of New Fentanyl Analogs. Front. Pharmacol. 2019, 10 (APR), 238. 10.3389/fphar.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekupec M. P.; Mansky P. A.; Baumann M. H. Misuse of Novel Synthetic Opioids: A Deadly New Trend. J. Addict. Med. 2017, 11 (4), 256–265. 10.1097/ADM.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Hosokawa A.; Bierly J. 11-Year Study of Fentanyl in Driving Under the Influence of Drugs (DUID) Casework. J. Anal. Toxicol. 2022, 46 (3), 337–341. 10.1093/jat/bkab049. [DOI] [PubMed] [Google Scholar]
- Kiely E.; Juhascik M. Fentanyl, Acetylfentanyl, and Carfentanil in Impaired Driving Cases: A Review of 270 Cases. J. Anal. Toxicol. 2021, 45 (9), 913–917. 10.1093/jat/bkab085. [DOI] [PubMed] [Google Scholar]
- Palamar J.; Salomone A.; Bigiarini R.; Vincenti M.; Acosta P.; Tofighi B. Testing Hair for Fentanyl Exposure: A Method to Inform Harm Reduction Behavior among Individuals Who Use Heroin. Am. J. Drug Alcohol Abuse 2019, 45 (1), 90–96. 10.1080/00952990.2018.1550652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton J.; Whitley P.; LaRue L.; Bundy W.; Dawson E.; Huskey A. Fentanyl Analog Positivity among Near-Real-Time Urine Drug Test Results in Patients Seeking Health Care. Drug Alcohol Depend. 2020, 217, 108264. 10.1016/j.drugalcdep.2020.108264. [DOI] [PubMed] [Google Scholar]
- Hwang C. S.; Smith L. C.; Natori Y.; Ellis B.; Zhou B.; Janda K. D. Efficacious Vaccine against Heroin Contaminated with Fentanyl. ACS Chem. Neurosci. 2018, 9 (6), 1269–1275. 10.1021/acschemneuro.8b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. S.; Smith L. C.; Natori Y.; Ellis B.; Zhou B.; Janda K. D. Improved Admixture Vaccine of Fentanyl and Heroin Hapten Immunoconjugates: Antinociceptive Evaluation of Fentanyl-Contaminated Heroin. ACS Omega 2018, 3 (9), 11537–11543. 10.1021/acsomega.8b01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks L. M.; Blake S.; Natori Y.; Ellis B.; Bremer P. T.; Janda K. D. A Highly Efficacious Carfentanil Vaccine That Blunts Opioid-Induced Antinociception and Respiratory Depression. ACS Chem. Biol. 2021, 16 (2), 277–282. 10.1021/acschembio.1c00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori Y.; Hwang C. S.; Lin L.; Smith L. C.; Zhou B.; Janda K. D. A Chemically Contiguous Hapten Approach for a Heroin-Fentanyl Vaccine. Beilstein J. Org. Chem. 2019, 15, 1020–1031. 10.3762/bjoc.15.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulima A.; Jalah R.; Antoline J. F. G.; Torres O. B.; Imler G. H.; Deschamps J. R.; Beck Z.; Alving C. R.; Jacobson A. E.; Rice K. C.; Matyas G. R. A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J. Med. Chem. 2018, 61 (1), 329–343. 10.1021/acs.jmedchem.7b01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R. C.; Whalen C.; Torres O. B.; Sulima A.; Bow E. W.; Komla E.; Beck Z.; Jacobson A. E.; Rice K. C.; Matyas G. R. Bivalent Conjugate Vaccine Induces Dual Immunogenic Response That Attenuates Heroin and Fentanyl Effects in Mice. Bioconjugate Chem. 2021, 32 (11), 2295–2306. 10.1021/acs.bioconjchem.1c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse B.; Wu M. M.; Gradinati V.; Kassick A. J.; Song D.; Jahan R.; Averick S.; Runyon S.; Comer S. D.; Pravetoni M. Efficacy and Selectivity of Monovalent and Bivalent Vaccination Strategies to Protect against Exposure to Carfentanil, Fentanyl, and Their Mixtures in Rats. ACS Pharmacol. Transl. Sci. 2022, 10.1021/acsptsci.1c00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCranor B. J.; Jennings L.; Tressler J.; Tuet W. Y.; DeLey Cox V. E.; Racine M.; Stone S.; Pierce S.; Pueblo E.; Dukes A.; Litvin S. R.; Leyden M. R.; Vignola J. N.; Pennington M. R.; Wong B. Assessment of Naloxone as a Therapeutic for Inhaled Carfentanil in the Ferret. Toxicol. Rep. 2020, 7, 1112–1120. 10.1016/j.toxrep.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcido D. D.; Koller A. C.; Genbrugge C.; Fink E. L.; Berg R. A.; Menegazzi J. J. Injury Characteristics and Hemodynamics Associated with Guideline-Compliant CPR in a Pediatric Porcine Cardiac Arrest Model. Am. J. Emerg. Med. 2022, 51, 176–183. 10.1016/j.ajem.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga H. A.; Lervik A.; Nordgreen J. Inhibition and Facilitation of Nociceptively Evoked Muscular Activity by Fentanyl or Dexmedetomidine in Isoflurane-Anaesthetized Pigs. Vet. Anaesth. Analg. 2021, 48 (2), 230–238. 10.1016/j.vaa.2020.09.007. [DOI] [PubMed] [Google Scholar]
- Baehr C.; Huseby Kelcher A.; Khaimraj A.; Reed D. E.; Pandit S. G.; AuCoin D.; Averick S.; Pravetoni M. Monoclonal Antibodies Counteract Opioid-Induced Behavioral and Toxic Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 2020, 375 (3), 469–477. 10.1124/jpet.120.000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.