Abstract
Background
Host–pathogen interactions can lead to dramatic changes in host feeding behaviour. One aspect of this includes self-medication, where infected individuals consume substances such as toxins or alter their macronutrient consumption to enhance immune competence. Another widely adopted animal response to infection is illness-induced anorexia, which is thought to assist host immunity directly or by limiting the nutritional resources available to pathogens. Here, we recorded macronutrient preferences of the global pest cockroach, Blatta orientalis to investigate how shifts in host macronutrient dietary preference and quantity of carbohydrate (C) and protein (P) interact with immunity following bacterial infection.
Results
We find that B. orientalis avoids diets enriched for P under normal conditions, and that high P diets reduce cockroach survival in the long term. However, following bacterial challenge, cockroaches significantly reduced their overall nutrient intake, particularly of carbohydrates, and increased the relative ratio of protein (P:C) consumed. Surprisingly, these behavioural shifts had a limited effect on cockroach immunity and survival, with minor changes to immune protein abundance and antimicrobial activity between individuals placed on different diets, regardless of infection status.
Conclusions
We show that cockroach feeding behaviour can be modulated by a pathogen, resulting in an illness-induced anorexia-like feeding response and a shift from a C-enriched to a more P:C equal diet. However, our results also indicate that such responses do not provide significant immune protection in B. orientalis, suggesting that the host’s dietary shift might also result from random rather than directed behaviour. The lack of an apparent benefit of the shift in feeding behaviour highlights a possible reduced importance of diet in immune regulation in these invasive animals, although further investigations employing pathogens with alternative infection strategies are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12862-022-02007-8.
Keywords: Self-medication, Macronutrient, Proteome, Anorexia, Cockroach, Immunity
Background
The animal immune system acts as a key interface between host and symbiont ecology [74]. In addition to the immune system, behavioural mechanisms have attracted increasing attention for their ability to coordinate host responses to infection [78, 98]. Behaviour is the primary means by which animals interact with the biotic environment, and its importance for a wide range of immune-related functions has recently witnessed a resurgence in research interest.
Hosts can respond behaviourally before infection has even taken place. This can include avoidance of pathogen transmission areas (e.g., defecation sites) and deterrence of disease vectors [30, 55]. Other prominent examples include activities falling within the category of ‘social immunity’, which among insects can include pathogen detection alarm behaviours [70]; grooming of conspecific group members [68, 71]; removal [5] or even destruction of infected individuals [17, 99]. Such mechanisms are well documented in many social insect lineages, where they contribute significantly to a number of prophylactic mechanisms operating within societies [16, 73]. Other prophylactic social behaviours include the collection of secondary antimicrobial compounds to prevent microbial growth in the nest environment [11, 77], in addition to the direct use—typically via feeding—of antimicrobials in both individual and transgenerational prophylaxis [18, 40, 47, 48, 53].
Once the infection has occurred, the first and principal line of defence is the immune system. Here, behavioural defensive adaptations can also play an important role in regulating or augmenting the response to infection. As with prophylaxis, the role of feeding behaviour has increasingly been viewed as a key mechanism by which animals can respond to infection [1]. Here, the selection of novel antimicrobial compounds, or the enrichment of specific dietary elements can be employed as therapeutic treatment against pathogens [18]. Fruit flies use ethanol therapeutically as well as prophylactically to combat parasitoid wasp infection [53] whereas parasitoid fly-infected Grammia caterpillars mix pyrrolizidine alkaloid-producing toxic plants into the normal diet to assist parasitoid clearance, which comes at the expense of body growth [80, 81, 85].
Infection-induced adaptive changes to feeding behaviour can also involve modifications to the quantity and composition of macronutrients in the diet. Anorexia is a well-documented response to infection in both vertebrates [38, 44] and invertebrates [4, 7] and is thought to assist hosts in limiting nutritional resources available to pathogens [43]. Anorexia may also help by activating components of the immune system that are enhanced under conditions of nutritional stress, such as autophagy [95, 96]. In recent years, the balance of macronutrients itself has been examined as a way for animals to regulate the response to infection. In particular, the proportion of protein (P) has been shown to be an important criterion in animal choice of diet following infection. In Spodoptera moths, larvae select a diet enriched in P following infection with a generalist Gram-positive bacterium and a host-specific DNA virus [45, 62, 63], leading to enhanced antimicrobial activity in both cases. By contrast, diets enriched in carbohydrate (C) were selected when Tenebrio beetles and Grammia caterpillars were infected with a rat tapeworm [61] and a parasitoid fly [50], respectively. In the latter study, this behaviour was also associated with an enhanced melanisation response.
The use of macronutrients by hosts to regulate immunity could in principle apply to any animal that is not an obligate food specialist. But less is known about the relationship between macronutrient diet choice and immunity outside of holometabolous insects. Holometabolous insects undergo complete metamorphosis consisting of distinct larval, a pupal and an adult winged phase, which are typically correlated with vastly different ecologies and corresponding physiological, morphological and immunological conditions [52]. By contrast, hemimetabolous insects undergo progressive molts where each larval instar closely resembles the adult [75]. Studies in both locusts and crickets have identified significant correlations between macronutrient intake and immune activity [66, 89], but these can result in contrasting effects on host resistance to pathogen infection [25, 87], pointing to a complex relationship between diet, immunity and infection in Orthoptera [88].
Among cockroaches, nutritional studies in Nauphoeta cinerea have found that, unlike other insects [36, 51], both sexes prefer a diet enriched in C [8]. Some studies on the invasive German cockroach, Blattella germanica, suggest an apparent robustness to nutritional imbalance and a rapid ability for recovery and dietary adaptation [67, 76], although see [37]. This ability may be linked to the fact that cockroaches harbour endosymbiotic bacteria in the fat body that can assist in storing excess nitrogen during over-consumption of P, which can then be redeployed when P is scarce [72]. Such traits make cockroaches an interesting target for research into the interaction between nutrition and immunity, but this topic has hitherto received relatively little attention.
We tackled this by examining the interaction between macronutrient feeding behaviour and immunity in the omnivorous oriental cockroach, Blatta orientalis. We investigated the macronutrient preferences of adult males in response to a range of sublethal immune challenges, before examining the impact of macronutrients on host survival, immune resistance and finally, the expression of the host’s proteome, which captures an additional aspect of the host’s immune response to a pathogen. We tested the hypothesis that B. orientalis males modulate macronutrient consumption in response to infection by upregulating the relative intake of P, in turn leading to improved host survival and an enhanced immune response.
Methods
Insects and bacteria
A breeding culture of sequential B. orientalis cohorts was established at the Federal Institute for Materials Research and Testing (BAM, Berlin, Germany) in June 2015, initially obtained from the collection at the Federal Environment Agency, Berlin, Germany, which consists of a mixed population of 4 independent genetic backgrounds maintained for 50 generations. Each generation consists of a minimum of 150 breeding pairs of cockroaches to minimize the effects of inbreeding. Each experimental cohort generation (comprising populations reared independently) was maintained for approximately 190 days in the dark at 26 °C and 50% humidity, from the day of egg-laying until disposal of older adults. Prior to being placed on experimental (artificial) diets, animals were reared on a mixture of 77.0% dog biscuit powder, 19.2% oat flakes and 3.8% brewer’s yeast and supplied with water ad libitum and weekly with apple and carrot slices. All experiments were conducted with adult males (2–3 weeks post final molt) to minimise changes in physiology associated with oogenesis. Each individual was used only once in each experiment. For the food choice experiment and the survival on enforced diets, individuals from 3 different cohorts were used. The generalist Gram-negative bacterial pathogen Pseudomonas entomophila (strain L48; DSM No. 28517) which is able to infect a variety of insect orders [65, 94] was obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures. Bacteria were stored at − 70 °C until use in experiments.
Artificial diets
The artificial diets used in this study are based on isocaloric diets, as described elsewhere [45, 63], which were slightly modified to suit cockroach needs: namely, a drying step was introduced at the end of the diet preparation as cockroaches were not able to eat wet food blocks. We employed diets containing 35% C and 7% P or vice versa, or an equal (E) diet containing 21% C and 21% P. The latter diet was selected for some assays because it resembles the composition preferred by cockroaches infected with a high sublethal dose of P. entomophila (Fig. 1D) The C portion consisted of sucrose while the P portion consisted of casein, peptone and albumin from eggs in a 3:1:1 ratio. Remaining ingredients are listed in Additional file 13: Table S1. Diet blocks of approximately 0.125 cm3 in size were dried at 50 °C for 2 days before being weighed and given to experimental cockroaches.
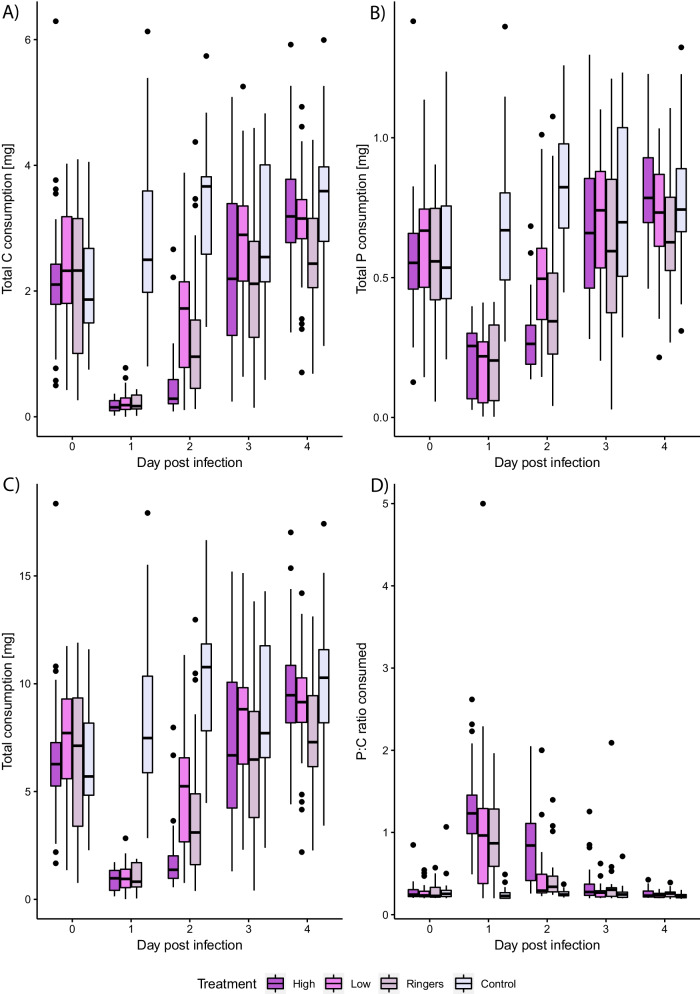
Fig. 1.
Effect of bacterial infection with P. entomophila (high load, low load), Ringer’s solution or no manipulation (control) of B. orientalis males on: A C consumption, B P consumption, C total consumption, D P:C ratio consumed. Note different scales used for total P- and C- consumption
Bacterial inoculation
About 200 µl of an overnight culture of P. entomophila was mixed in 10 ml fresh liquid medium (according to DSMZ instructions) and incubated at 28 °C and 140 rpm to an OD600 of 0.55, representing 1.5 × 108 CFUs per ml. The desired concentrations of bacteria were subsequently obtained by diluting bacteria in insect Ringer’s solution (0.024 g calcium chloride, 0.021 g potassium chloride, 0.01 g sodium hydrogen carbonate, 0.45 g sodium chloride, 200 ml distilled water). Cockroaches were anaesthetised with CO2, abdomens swabbed with 70% ethanol, then injected with 2 µl of bacterial solution directly into the hemocoel using a glass capillary needle inserted between the 3rd and 4th abdominal segment. Sublethal infections (high: 5.8 × 105 CFUs/2 µl, low: 5.8 × 103 CFUs/2 µl) and lethal (4.0 × 106 CFUs/2 µl) doses were determined in pre-experiment injection assays.
Diet choice following sublethal infection
From each of 3 cohorts, 40 B. orientalis males (120 in total) were given free choice of macronutrients by placing them together with 1 block of known weight of each P-rich and C-rich diet. Individuals were kept for 3 days to accustom them to artificial diets, and to obtain a baseline P:C ratio preference. Thereafter, food blocks were collected, placed at 50 °C until completely dry, and then their weight loss was determined, equating to the amount eaten by the cockroach. Experimental cockroaches were assigned randomly to one of the following sublethal treatments (40 per treatment): (1) High infection (injected 5.8 × 105 P. entomophila CFUs); (2) Low infection (injected 5.8 × 103 P. entomophila CFUs); (3) Wounding control (injected Ringer’s solution); (4) Unmanipulated control. Cockroaches were then placed on new food blocks of both diets of known weight. The blocks were replaced daily for 4 days and their loss of weight was again determined after drying at 50 °C.
Survival on enforced diet
From each of 3 cohorts, 10 B. orientalis males were placed on P-rich diet (35% P; 7% C) and another 10 were placed on C-rich diet (7% P; 35% C). All individuals were supplied with water ad libitum. Survival was checked twice weekly; food blocks and water were changed once a week over the period of 150 days.
Survival on enforced diet following lethal infection
Two hundred and seventy B. orientalis males were assigned to one of the following treatments: (1) 150 individuals: Infection (injected 9.0 × 105 P. entomophila CFUs); (2) 60 individuals: Wounding control (injected Ringer’s solution); (3) 60 individuals: Unmanipulated control. A third of the individuals from each treatment were immediately randomly assigned to either a P- (35% P; 7% C), C-enriched (7% P; 35% C) or a E (21% P; 21% C) artificial diet and supplied with water ad libitum. Survival of each individual was recorded every 2 h for 139 h with overnight intervals of 8 h. The experiment was conducted twice, and the data were combined for subsequent analysis (N = 540).
Hemolymph collection
Hemolymph for the bacterial growth inhibition assay and proteomic analysis (below) was collected by cutting the first two leg pairs of cockroaches that had been pre-chilled on ice for 15 min. They were then placed head-down into a spin-column (Sigma-Aldrich) in a 1.5 ml tube containing approximately 10 μg propylthiouracil (to inhibit phenol-oxidase activity), and centrifuged at 500 g for up to 5 min or until at least 10 μl of hemolymph were collected. The entire operation was carried out in a pre-cooled centrifuge at 2 °C.
Bacteria growth inhibition assay
In an initial bacterial growth inhibition assay, 180 B. orientalis males were equally assigned to the following treatments: (1) bacteria challenge (injected 5.8 × 105 P. entomophila CFUs); (2) Wounding control (injected Ringer’s solution); (3) Unmanipulated control. A third of the individuals from each treatment was randomly assigned to either a P- (35% P; 7% C), C-enriched (7% P; 35% C) or a E (21% P; 21% C) artificial diet and supplied with water ad libitum. After 24 h the hemolymph of each individual was collected as described in the hemolymph collection section and the hemolymph from 5 individuals per treatment was pooled (resulting in 4 pools per treatment). Pools were stored at -70 °C until needed. A second bacteria growth inhibition assay was conducted on a subset of treatments as an independent validation of the first assay. Methods were identical, except 120 B. orientalis males were challenged with bacteria (injected 5.8 × 105 P. entomophila CFUs), with half of the individuals being randomly assigned to either a P- (35% P; 7% C) or C-enriched (7% P; 35% C) artificial diet, and hemolymph each from 10 individuals being pooled per treatment (resulting in 6 pools per treatment).
In both assays, bacterial growth inhibition of the cockroach hemolymph was measured using a plate reader assay. First, 10 μl Mueller–Hinton broth were added to each well of a 384-well polypropylene plate. Then 10 μl hemolymph was loaded in the second and the ninth column of the plate. One of these wells contained the hemolymph of one pool of animals (in total 36 wells loaded with hemolymph). Four wells in the first column which did not contain hemolymph (replaced with an equal amount of Mueller–Hinton broth) served as the negative control. A five-step serial dilution of the hemolymph was performed (with the last 10 μl being discarded) and 10 μl P. entomophila in Mueller–Hinton broth with an OD600 of 0.005 was added to each well containing hemolymph as well as to another four wells in the ninth column not containing hemolymph, which served as a positive control for unsuppressed bacterial growth. OD600 was measured in a plate reader (BioTek) every 10 min for 16 h at room temperature.
Proteomic analysis
We were unable to detect any significant effect of an equal diet on hemolymph antimicrobial activity when compared with other diets, regardless of infection treatment (Fig. 3, Additional file 13: Table S6). Therefore, we restricted our proteomic analysis to a comparison of the most divergent bacterial-killing diets: P-rich vs. C-rich following sublethal challenge. One hundred and twenty B. orientalis males were immune-challenged by injecting 2 μl Ringer’s solution containing 5.8 × 105 P. entomophila CFUs. Half were assigned to the P- (35% P; 7% C) and the other half to the C-enriched (7% P; 35% C) artificial diet and supplied with water ad libitum. Twenty-four hrs later the hemolymph of each individual was collected as described in the hemolymph collection section and stored at − 70 °C until needed. This time-point matched the sampling point of the antibacterial assay and was selected to coincide with the peak of infection. The rationale being that for the dietary shift to be relevant for immune activity it must take effect by this point. A detailed description of protein sample preparation and liquid chromatography-mass spectrometry and data processing is described in Additional file 13. We employed de novo transcriptome data from He et al. [32] to generate a peptide database for B. orientalis (Additional file 1: Data sheet 1). Raw data were processed and annotated as described elsewhere [31] (Additional file 13). For proteomic analysis, protein identification and label-free quantification was performed using MaxQuant (v1.6.0.1) with Andromeda search engine [14, 15, 93]. Raw data were matched against an in-house protein database of B. orientalis created by de novo transcriptome sequencing (see above). Trypsin was selected as enzyme allowing a maximum of two missed cleavages. The minimum peptide length was set to 7 amino acids and the false discovery rate for peptide and protein identification was set to 0.01.
Fig. 3.
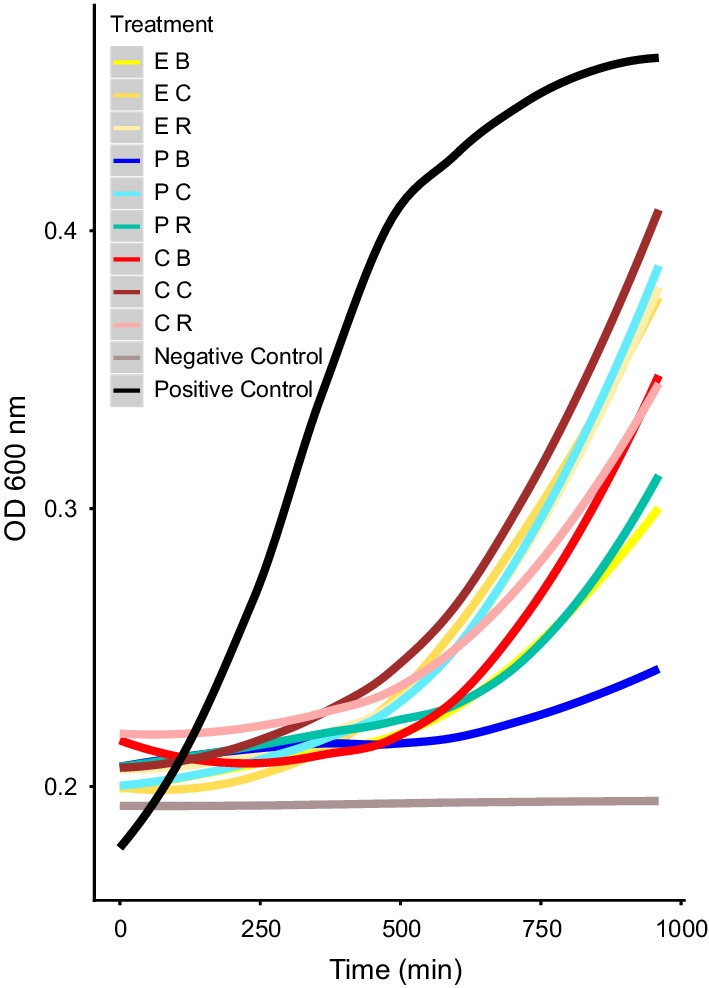
Impact of diet on B. orientalis hemolymph growth inhibition of P. entomophila in vitro (1:4 dilution). Immune-challenged individuals on P-rich (P B), C-rich (C B) equal (E B) diet. Ringer’s solution injected (wounded) individuals on P-rich (P R), C-rich (C R) or equal (E R) diet. Control (unmanipulated) individuals on P-rich (P C), C-rich (C C) or equal (E C) diet. A bacterial solution without hemolymph served as the positive control and a solution containing only the growth medium (Mueller Hinton) served as the negative control
Statistical analysis
All statistical analyses were carried out in R v4.0.3 [64]. Testing for normality was performed using the ksnormal function of the wrappedtools package v0.3.11 [10]. P:C ratios, the amounts of P and C eaten as well as total consumption differences between treatments for the first day following infection were analysed using Bonferroni-corrected Wilcoxon rank sum tests.
The food-choice data were analysed using a generalized linear mixed model (GLMM) with an underlying beta family distribution. Analyses were run in the glmmADMB package v0.8.3.3 [23, 83] in conjunction with the R2admb package v0.7.16.2 [7]. GLMMs examined whether a response variable consisting of proportion of P consumed (amount of P eaten divided by the amount of total diet eaten) or proportion of C (amount of C eaten divided by the amount of total diet eaten) was influenced by treatment (high infection; low infection; wounded; and unmanipulated) and day post infection as well as an interaction between treatment and day. Minimal adequate models were derived by stepwise-model simplification and comparison via ANOVA. Individual and cohort were treated as random effects to account for multiple measurements and origin. Comparisons among treatment levels were carried out with post-hoc Tukey tests using a Bonferroni correction, using package multcomp v1.4-15 [34]. Five individuals were removed prior to analysis due to the presence of fungal growth on the artificial diet blocks.
The effect of treatment and diet on survival was analysed using Cox proportional hazard models with the package coxme 2.2-16 [91]. Median survival time for each treatment was calculated using the survminer package v0.4.8 [41, 42]. Because control data in the survival on enforced diet following infection experiment were right-censored, we uncensored one randomly selected individual from each treatment, following Tragust et al. [92]. Owing to the high number of comparisons between treatment levels in this experiment, we conducted post-hoc Tukey tests with Bonferroni or false discovery rate (FDR) corrections and report the results of both methods. Bacterial growth inhibition data was only analyzed for the 1:4 hemolymph dilution since undiluted and highly diluted treatments were either preventing bacterial growth completely or not at all using R package growthcurver 0.3.1 [86] using default parameters. Empirical area under the curve (eAUC) values were analyzed using t-tests in the R package rstatix v0.6.0 [41, 42]. Due to the high number of post hoc comparisons, pairwise t-tests were again conducted with Bonferroni as well as FDR corrections. As before, both sets of p-values are reported. In a second analysis bacterial growth inhibition data were combined for a subset of treatments (N = 10 replicates per treatment, Pinfected vs. Cinfected) from two independent assays, using a two-way ANOVA to examine the eAUC value, with an interaction between treatment and assay.
Results
Diet choice following sublethal infection
Individual cockroaches ate on average 0.58 mg P and 2.24 mg C under unmanipulated conditions, and this remained stable throughout the experiment. Conversely, in all manipulated groups, total food as well as P and C consumption varied significantly over the course of the experiment (Fig. 1A–C). The total amount eaten was reduced in all challenged treatments compared to unmanipulated cockroaches on the first day post-infection (p.i.), but did not differ significantly between manipulated treatments (Wilcoxon rank sum test: high vs. low: W = 429, p > 0.1; high vs. wounded: W = 384.5, p > 0.1; high vs. unmanipulated: W = 0, p < 0.001; low vs. wounded: W = 413.5, p > 0.1; low vs. unmanipulated: W = 0, p < 0.001; wounded vs. unmanipulated: W = 0, p < 0.001). This pattern was replicated in the consumption of P on the first day p.i. (Wilcoxon rank sum test: high vs. low: W = 542.5, p > 0.1; high vs. wounded: W = 452, p > 0.1; high vs. unmanipulated: W = 15, p < 0.001; low vs. wounded: W = 388, p > 0.1; low vs. unmanipulated: W = 12, p < 0.001; wounded vs. unmanipulated: W = 17, p < 0.001) and the consumption of C on the first day p.i. (Wilcoxon rank sum test: high vs. low: W = 382.5, p > 0.1; high vs. wounded: W = 334, p > 0.1; high vs. unmanipulated: W = 0, p < 0.001; low vs. wounded: W = 413.5, p > 0.1; low vs. unmanipulated: W = 0, p < 0.001; wounded vs. unmanipulated: W = 0, p < 0.001). However, by the 2nd day, consumption across all manipulated groups began to recover, reaching pre-treatment levels by the 4th day p.i.
Before wounding or infection, cockroaches of all treatments preferred a median P:C ratio of approximately 1:4.24 (Fig. 1A). The unmanipulated animals consumed this ratio over the course of the experiment. By contrast, highly infected individuals changed to a P:C ratio of approximately 1.23:1 whereas low infected and wounded cockroaches shifted to an intermediate ratio of 1:1.04 and 1:1.15 P:C on the first day p.i., respectively (Wilcoxon rank sum test: high vs. low: W = 595, p > 0.1; high vs. wounded: W = 571, p > 0.1; high vs. unmanipulated: W = 810, p < 0.001; low vs. wounded: W = 412.5, p > 0.1; low vs. unmanipulated: W = 695.5, p < 0.001; wounded vs. unmanipulated: W = 713.5, p < 0.001). All manipulated groups returned to baseline P:C ratios by day 4 p.i. (Additional file 2: Data sheet 2, Additional file 13: Table S2).
We then carried out GLMMs to explore food consumption differences between treatments over the course of the experiment. Final minimal GLMMs consisted of the fixed terms treatment and day without an interaction since the model with a treatment × day interaction did not significantly improve the model (ANOVA for model comparison, p > 0.1). Cockroaches that were wounded differed significantly from unmanipulated cockroaches in their consumed P proportion following treatment (mean values on first day p.i. are given) (P proportion chosen: 0.190 vs. 0.084, wounded vs. unmanipulated, respectively: z = − 6.132 p < 0.001), as did cockroaches infected with a high (P proportion chosen: 0.228 vs. 0.084, high vs. unmanipulated respectively: z = − 13.062, p < 0.001) or low bacterial dose (P proportion chosen: 0.187 vs. 0.084, low vs. unmanipulated respectively: z = − 5.332, p < 0.001) (Additional file 13: Table S3, Fig. S1). Cockroaches infected with a high bacterial dose also consumed a higher proportion of P compared to individuals exposed to both a low bacterial dose (P proportion chosen: 0.228 vs 0.187, high vs. low respectively: z = − 6.258, p < 0.001) or to wounding (P proportion chosen: 0.228 vs. 0.190, high vs. wounded respectively: z = − 4.786, p < 0.001). However, individuals that were wounded or were infected with a low bacterial dose did not consume a significantly different proportion of P to each other (P proportion chosen: 0.187 vs. 0.190, low vs. wounded respectively: z = 1.038, p = 1.000). Concerning the proportion of C, the pattern is the same (Additional file 13: Table S3, Fig. S2).
Survival on enforced diet without infection
The median (50%) survival time for B. orientalis males placed on a P-rich diet was 82 days, whereas the mortality of males placed on a C-rich diet did not exceed 30% throughout the experiment (150 days) (Fig. 2A) (Additional file 3: Data sheet 3). By the end of the experiment males restricted to P-rich diet showed a significantly higher mortality (86.44%) compared to those on C-rich diet (27.59%; Cox proportional hazard regression P vs. C: Hazard ratio = 5.73, z = 5.974, p < 0.001), although an overt increase in mortality of males restricted to a P-rich diet is only observable after approximately 40 days (Fig. 2A).
Fig. 2.
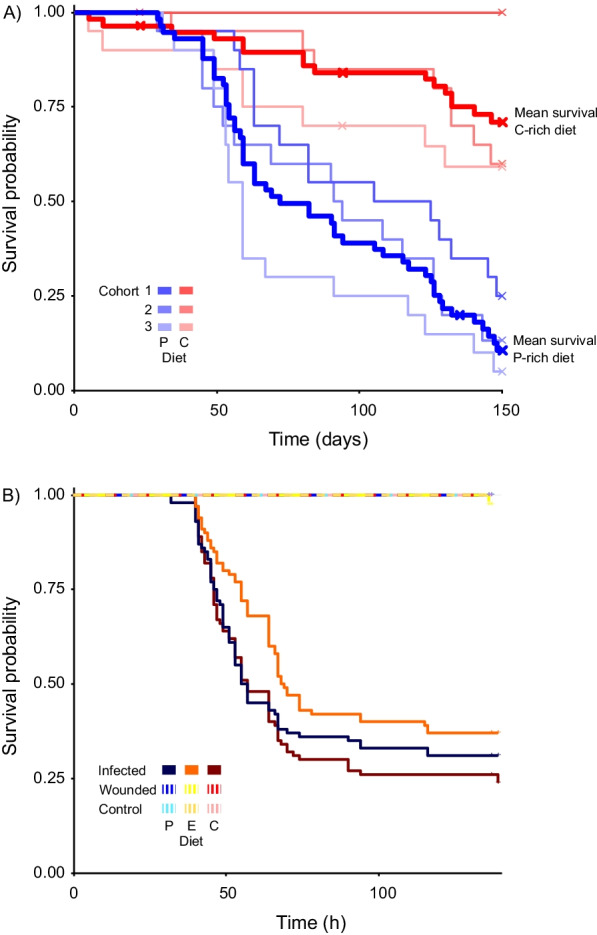
Kaplan–Meier survival curves of: A Unmanipulated B. orientalis males restricted to P-rich (35% protein and 7% carbohydrate) or C-rich (7% protein and 35% carbohydrate) diets. Survival data for three independent cohorts (1–3) for P- and C-rich diets are given in blue and red respectively, with mean population survival across cohorts on each diet indicated by a thick bold line. Note the long period at the beginning of the experiment where no clear survival differences between diets are observable. B B. orientalis males restricted to P-rich (35% protein and 7% carbohydrate) (blue line), C-rich (7% protein and 35% carbohydrate) (red line, or E (21% protein and 21% carbohydrate) (yellow line) diet following injection with an LD50 of P. entomophila (infected), Ringer’s solution (wounded) or unmanipulated (control)
Survival on enforced diet following infection
In our test of the effect of dietary composition on survival following lethal infection, we found that cockroaches on all diets began to die at 40 or 41 h after injection (Fig. 2B). This included individuals on the E diet, that is the diet which most closely resembled the ratio consumed by cockroaches following sublethal infection. The median survival time for infected B. orientalis males was 56, 57 and 68.5 h on P-rich, C-rich and E diets, but the effect of diet on survival following infection was not significant when the Bonferroni correction was implemented (Cox proportional hazard regression: Pinfected vs. Cinfected: Hazard ratio = 0.89, z = − 0.723, p = 1.000; Einfected vs. Cinfected: Hazard ratio = 0.66, z = 2.504, p = 0.443; Einfected vs Pinfected: Hazard ratio = 0.73, z = 1.765, p = 1.000) (Additional file 4: Data sheet 4, Additional file 5: Data sheet 5; Additional file 13: Table S4). These findings were similar when using FDR, except that survival was significantly higher in infected cockroaches exposed to an E- vs. a C-diet (Hazard ratio = 0.66, z = 2.504, p = 0.023) (Additional file 13: Table S5). Only one control individual (wounded, E-diet) died during the course of the experiment.
Bacteria growth inhibition assay
The inhibitory effect of male B. orientalis hemolymph (N = 4 per dilution per treatment) on bacterial growth was not diet-dependent, either within or between treatments (bacteria challenged, wounded or unmanipulated) (Fig. 3) (Additional file 6: Data sheet 6, Additional file 7: Data sheet 7). This was reflected in the non-significant differences of the t-test in the suppression of bacterial growth between all dietary pairwise comparisons, as expressed by eAUC values (Pinfected vs. Cinfected: t = 0.902, df = 3.212, p > 0.1; Einfected vs. Cinfected: t = 0.081, df = 5.491, p > 0.1; Einfected vs. Pinfected: t = 1.085, df = 3.396, p > 0.1; Pwounded vs. Cwounded: t = 0.494, df = 3.420, p > 0.1; Ewounded vs Cwounded: t = − 0.471, df = 4.845, p > 0.1; Ewounded vs. Pwounded: t = 1.643, df = 4.177, p > 0.1; Punmanipulated vs. Cunmanipulated: t = 0.332, df = 4.362, p > 0.1; Eunmanipulated vs. Cunmanipulated: t = 0.144, df = 5.175, p > 0.1; Eunmanipulated vs. Punmanipulated: t = 0.066, df = 3.612, p > 0.1.) Some but not all growth curves were significantly different to either the negative or the positive control. No clear pattern was observable between dietary treatments and controls, except that when using a Bonferroni correction, only hemolymph from cockroaches fed on P-diets (bacteria challenged, wounded and unmanipulated), in addition to the negative control, differed significantly from the positive control (Additional file 13: Table S6). Combining a subset of these eAUC values with a supplementary antibacterial assay of Pinfected vs. Cinfected (N = 10 replicates per treatment) yielded a similarly non-significant result (two-way ANOVA, F = 0.564, df = 1, p > 0.1) (Additional file 8: Data sheet 8, Additional file 9: Data sheet 9, Additional file 13: Table S7, Fig. S3).
Proteomic analysis
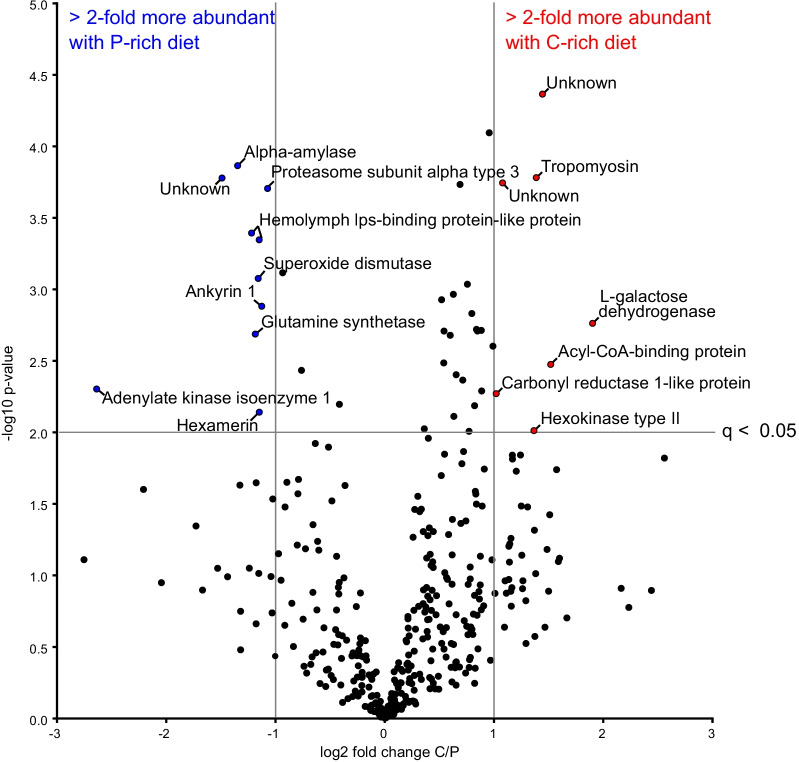
Using LC–MS analysis in combination with label-free quantification, 387 proteins were identified and quantified in the hemolymph of infected B. orientalis males fed on a P-rich vs. a C-rich diet (N = 6 per treatment) (Additional file 10: Data sheet 10). Overall, apolipophorin was the most abundant protein making up approximately 70% of the whole hemolymph protein content. Other highly abundant proteins were transferrin, gelsolin, heterochromatin-associated protein MENT and an insulin-like growth factor-binding protein complex. We identified 17 proteins that showed significant relative changes in abundance following diet treatment (Fig. 4 and Additional file 13: Table S8). Infected individuals on a C-rich diet were significantly more abundant for hexokinase type II, which is involved in carbohydrate metabolism (glycolysis) [100], in addition to carbonyl reductase 1-like protein, which is involved in NADPH-dependent reduction of active substrates including endogenous and xenobiotic carbonyl compounds [33]. Additionally, tropomyosin which is a calcium-dependent regulator of muscle contraction [60], and acyl-CoA-binding protein, which carries out lipid-binding transport and suppresses glucose-induced insulin secretion [21, 57] were more abundant. Furthermore, a l-galactose dehydrogenase-like protein was more abundant but its function is not known in insects. Conversely, infected individuals on a P-rich diet were significantly enriched for alpha-amylase, which is involved in carbohydrate metabolism [90] and proteasome subunit alpha type 3, which is involved in protein degradation [69]. Additionally, enhanced levels of hemolymph lipopolysaccharide-binding protein-like proteins (2 isoforms), which bind carbohydrates (foreign particles) [39] and extracellular superoxide dismutase, which carries out superoxide metabolic processing [22] were detected. Furthermore, greater abundance of glutamine synthetase, a adenylate kinase isoenzyme 1 and a hexamerin was detected. Glutamine synthetase is involved in glutamate and glutamine catabolism and biosynthesis [84] while adenylate kinase isoenzyme 1 and hexamerin are associated with ATP metabolism [24] and amino acid and energy storage, respectively [9]. Ankyrin 1 was also found to be more abundant, although its function in insects remains unclear.
Fig. 4.
Effect of diet on abundance of male B. orientalis hemolymph proteins following bacterial challenge (high dose). Points in blue and red reflect proteins that are significantly (> 2) more abundant in P- and C-rich diets respectively
Discussion
Under normal conditions, extensive P consumption shortens the lifespan of many insects including ants, honeybees and flies [13, 19, 20, 26, 46, 59], a finding that is corroborated in our and other studies of cockroaches [28, 29]. Here, we find that male B. orientalis cockroaches showed 45% higher mortality (Fig. 2A) when restricted to a P- vs. a C-rich diet. One explanation for this consistent observation across study organisms is that elevated levels of P increase TOR signalling. TOR serves as a nutrient sensor linked to macronutrient intake and metabolism, causing a broad anabolic response that is life-shortening over the long term (reviewed in [79]. Other explanations could relate to the toxic effects of breaking down nitrogenous products, and the enhanced production of mitochondrial radical oxygen species, DNA and protein oxidative modifications, membrane fatty acid composition and mitochondrial metabolism [79]. The higher abundance of extracellular superoxide dismutase in cockroach males fed on a P-rich diet (Fig. 4, Additional file 13: Table S8) supports this explanation. Furthermore, the overrepresentation of proteins participating in carbohydrate and protein metabolism in C- vs. P-rich diets, respectively, demonstrate that the diets altered cockroach physiology in the expected direction. For example, the higher abundance of alpha-amylase in the hemolymph of B. orientalis males feeding on P-rich diet shows these individuals were metabolizing lower quantities of C. Alpha-amylase is thought to be involved in the breakdown of glycogen, which is the major glucose storage compound in animals. It is employed if not enough C is present in the diet [54].
Unsurprisingly, male cockroaches consumed low amounts of P under normal conditions (1:4.24 P:C). This is in line with the cockroach N. cinerea, where males preferred a similarly C-skewed diet of 1:4.8 (P:C) [8]. Data also indicate that B. germanica typically prefers a C-enriched diet, and the degree of C-skew appears to be highly sex-dependent in this species [35, 37]. In our study, the clear preference for C shifted significantly following infection. As with caterpillars [63], highly infected male cockroaches increased the ratio of P consumed. Furthermore, cockroaches appeared to adapt their feeding behaviour to the severity of the immune challenge. Lowly infected and wounded (Ringer-injected) individuals consumed an intermediate (approximately uniform) P:C ratio and their food consumption returned to normal sooner after challenge compared to highly infected individuals, which shifted to the most P-enriched diet and displayed the longest delay in returning to normal dietary consumption. It is interesting to note that the observed feeding responses were transient across all challenge treatments, with most individuals returning to a normal dietary intake 72–96 h after injection. Transience is likely correlated with the period of acute bacterial infection, although wounding itself also elicited a similar response to lowly infected individuals, suggesting a generalized precautionary host response to challenge. Together, our data indicate that B. orientalis males are able to quantitatively regulate their behavioural response to infection and rapidly return to a normal feeding regime. Additionally, our findings suggest host-driven adaptation as opposed to pathogen manipulation because wounded individuals also reduced their C intake. Wounding elicits a localized immune response in insects [27], suggesting a form of prophylactic behaviour since it is likely that microbes can enter the hemolymph via damaged cuticle [82].
In contrast to Spodoptera exempta caterpillars and other organisms which can modulate their immune response with diet, changes in cockroach dietary consumption following infection did not greatly influence any of the immune parameters we measured. In caterpillars, a shift from a C- to a P-biased diet following Bacillus subtilis (Gram-positive) or baculovirus infection led to an increase of antibacterial and phenol-oxidase activity and hemocyte density and resulted in higher survival [62, 63]. By contrast, a switch to a protein enriched diet did not have a major influence on male B. orientalis hemolymph antimicrobial activity or survival, nor have a substantial impact on the synthesis of induced immune-related proteins. We note, however, that two hemolymph lipopolysaccharide-binding protein isoforms, which may play a role in pathogen recognition by binding foreign particles [39] were more abundant in the hemolymph of P-rich fed infected cockroaches. Furthermore, we observed some evidence for reduced bacterial growth in hemolymph extracted from P-fed cockroaches (compared to the positive control), suggesting that dietary protein may confer some inhibitory effect on bacterial proliferation. However, this effect was not observed with the E-diet (the dietary blend consumed by males following infection), nor was this pattern consistently observed across post-hoc methods. A final potential caveat to consider here is that due to the use of phenylthiourea, this assay could not measure PO activity directly. On the other hand, no significant effect of diet was observed between any of the challenge treatments, and although we found that survival following infection was significantly higher in cockroaches fed on an E- vs. a C-diet, this effect was not consistent across correction methods, and was not corroborated by other dietary comparisons, as might be expected (e.g., E- vs. P-diet, or P- vs. C-diet).
Overall, our findings suggest that a shift to a protein enriched diet could have a minor influence on B. orientalis immunity, but that in general, the behavioural changes adopted by this cockroach following direct injection with P. entomophila are unable to substantially alter infection outcome. However, the longer-term consequences of P on B. orientalis immunity, including over the course of development, remain to be investigated. Entomopathogenic pathogens that act more slowly on the host should also be examined in this context. Studies in Orthoptera indicate that an enforced P-rich diet can enhance immune activity both over ontological time and in the short term. However, the benefits of P for host survival after infection are conflicting and appear to depend significantly on host and pathogen identity [25, 87]. Graham et al. [25] found that locusts feeding on a C-enriched diet were more resistant to fungal infection, even though enforced consumption of P enhanced several immune parameters, including antibacterial activity. But this outcome may be due to the metabolic requirements of the pathogen in question [87]. A point here is that dietary modulation during illness could still be selected for even if it only helps against some pathogens. With respect to host physiology, a recent study suggests that a diet enriched in P may protect against infection simply via the modulation of hemolymph osmolarity [97]. A hypothesis to explore here would be whether natural variation in hemolymph osmolarity might explain the relative (in) effectiveness of P dietary manipulation in different insects following bacterial infection. Some evidence shows that hemolymph osmolarity is higher in cockroaches compared with other insects, including lepidopterans, potentially as a result of different feeding habits [56], although this requires further testing in a broader spectrum of insect species. Similarly, it would be important to explore the feeding shifts of a greater diversity of hemimetabolous groups, including cockroaches, under challenge from a range of pathogenic microbes to understand whether the patterns we observed in B. orientalis are associated with specific adaptations such as extreme omnivory and/or endosymbiosis.
Taken together, our results suggest that B. orientalis males may not be able to effectively self-medicate against P. entomophila using macronutrients, but that they do engage in a typical anorexia response, as has been shown in macronutrient self-medication in caterpillars [4, 62, 63]. Illness-induced anorexia offsets physiological trade-offs between launching immune responses and food digestion. A previous study demonstrated that crickets reduce their food intake, especially for lipids, following infection with the bacterium Serratia marcescens [2]. High hemolymph lipid levels are associated with decreased concentrations of monomeric apolipophorin III, a lipid transporter, and higher susceptibility to S. marcescens infection [3]. In other insects, anorexia can have a direct impact on immunity. For example, in Drosophila, starvation can modify AMP production and lead to reduced melanisation [7].
The apparent lack of a link between macronutrient dietary selection and male cockroach immunity is unexpected. One possible explanation is that future food availability and quality may be less predictable in omnivorous pest organisms like cockroaches [67] and therefore, a beneficial diet might not always be accessible, therefore making such a link unreliable. A recent genomic study reports major expansions of cockroach gene families linked to chemoreception, detoxification and innate immunity [49], indicating that adaptations in these pathways permit cockroaches to thrive in unpredictable, antigen-rich environments. Indeed, while cockroach survival was reduced on an enforced P-rich diet, a negative effect could only be observed well over 40 days after exposure, suggesting that although protein is generally avoided by B. orientalis adult males, its consumption can be tolerated for long periods of time. Cockroaches are known to tolerate high levels of P consumption [12] and in such extreme omnivores, there could be an advantage to reducing regulatory interactions between host diet and immunity. This ability could be mediated by the presence of the endosymbiont Blattabacterium, which may also be able to help the host store and catalyze excess nitrogen [58].
Conclusions
We find that B. orientalis males modulate their macronutrient feeding behaviour following infection by dramatically reducing food intake and simultaneously reducing carbohydrate over protein intake. We also show that a P-rich diet eventually leads to significantly reduced lifespan, and that male cockroaches prefer a C-rich diet under normal conditions. To our surprise, the observed behavioural response to immune challenge did not substantially influence the antimicrobial activity or proteomic profile of host immunity. Our findings support the concept of a generalized host-directed response to microbial challenge in cockroaches based on anorexia and the limitation of C intake. In this scenario, the observed change to a more equal ratio of P:C may reflect a shift towards a severely reduced baseline level of random feeding rather than a directed shift towards a higher ratio of consumed P, although this hypothesis requires additional testing. Such a response may be beneficial to the host, but perhaps primarily as a means of avoiding contaminated food and reducing pathogen access to resources, rather than facilitating crosstalk with the immune system. From an evolutionary perspective, this could be the result of adaptations to detoxification, endosymbiont-mediated metabolism and innate immunity combining to enhance cockroach survival in antigen-rich and nutritionally diverse environments. Overall, our study highlights the importance of understanding variation in natural diet, physiology and ecology when exploring the link between nutrition and animal immunity.
Supplementary Information
Additional file 1. Protein database used for the proteomic analyses of the cockroach hemolymph.
Additional file 2. Data, diet choice following sublethal infection experiment.
Additional file 3. Data, survival on enforced diet experiment.
Additional file 4. Data, survival on enforced diet experiment after infection.
Additional file 5. Data, survival on enforced diet experiment after infection, plus deaths.
Additional file 6. Data, bacterial growth inhibition experiment.
Additional file 7. Results of the growthcruver analysis.
Additional file 8. Data, bacterial growth inhibition experiment (combined dataset) (Supplementary Fig. 3).
Additional file 9. Results of the growthcruver analysis (combined dataset).
Additional file 13. Supplementary File (Supplementary Tables andFigures).
Acknowledgements
We thank J. Rolff for providing useful advice and technical support.
Abbreviations
- AMP
Antimicrobial peptide
- CFUs
Colony-forming units
- DSMZ
German collection of microorganisms and cell cultures
- LC–MS
Liquid chromatography–mass spectrometry
- MS
Mass spectrometry
- OD600
Optical density at 600 nm
Author contributions
DPM conceived and coordinated the study; TS designed and conducted the experiments together with MAEM, SJ, VP, RB and MJJ; TS and DPM wrote the manuscript; SH and PRJ conducted the proteomic database preparation by de novo transcriptome sequencing; ARR guided the sample preparation for the proteomic analysis; BK and CW conducted LC–MS/MS and corresponding data analysis. All authors contributed to drafting and revising the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. S.H. was supported by the Chinese Scholarship Council and D.P.M. was supported by a seed-funding Grant provided by the Freie Universität Berlin and grant MC 436/6-1 from the Deutsche Forschungsgemeinschaft (DFG). We also acknowledge assistance of the Core Facility BioSupraMol supported by the DFG.
Availability of data and materials
The short read data used to generate the Blatta orientalis transcriptome are available on the SRA (ID: SRX8891863, part of Bioproject PRJNA635910). All other data generated or analyzed during this study are included in this published article and its additional information files.
Declarations
Ethics approval and consent to participate
No ethical guidance or approval was required for working with Blatta orientalis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thorben Sieksmeyer, thorbensieksmeyer@googlemail.com.
Shulin He, Email: shulinhe@hotmail.com.
M. Alejandra Esparza-Mora, Email: alejandra.esparza@fu-berlin.de.
Shixiong Jiang, Email: shixiong.jiang@fu-berlin.de.
Vesta Petrašiūnaitė, Email: vesta.petrasiunaite@gmail.com.
Benno Kuropka, Email: kuropka@zedat.fu-berlin.de.
Ronald Banasiak, Email: Ronald.Banasiak@bam.de.
Mara Jean Julseth, Email: mara-j@posteo.de.
Christoph Weise, Email: dada@zedat.fu-berlin.de.
Paul R. Johnston, Email: paul.johnston@fu-berlin.de
Alexandro Rodríguez-Rojas, Email: a.rojas@fu-berlin.de, Email: alexandro.rojas@vetmeduni.ac.at.
Dino P. McMahon, Email: dino.mcmahon@fu-berlin.de
References
- 1.Abbott J. Self-medication in insects: current evidence and future perspectives. Ecol Entomol. 2014;39:273–280. doi: 10.1111/een.12110. [DOI] [Google Scholar]
- 2.Adamo SA, Bartlett A, Le J, Spencer N, Sullivan K. Illness-induced anorexia may reduce trade-offs between digestion and immune function. Anim Behav. 2010;79:3–10. doi: 10.1016/j.anbehav.2009.10.012. [DOI] [Google Scholar]
- 3.Adamo SA, Roberts JL, Easy RH, Ross NW. Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J Exp Biol. 2008;211:531–538. doi: 10.1242/jeb.013136. [DOI] [PubMed] [Google Scholar]
- 4.Adamo SA, Fidler TL, Forestell CA. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav Immun. 2007;21:292–300. doi: 10.1016/j.bbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Armitage SA, Fernandez-Marin H, Boomsma JJ, Wcislo WT. Slowing them down will make them lose: a role for attine ant crop fungus in defending pupae against infections? J Anim Ecol. 2016;85:1210–1221. doi: 10.1111/1365-2656.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolker B. Using AD Model Builder and R together: getting started with the R2admb package. R Package Version 0.7.16. 2017.
- 8.Bunning H, Bassett L, Clowser C, Rapkin J, Jensen K, House CM, Archer CR, Hunt J. Dietary choice for a balanced nutrient intake increases the mean and reduces the variance in the reproductive performance of male and female cockroaches. Ecol Evol. 2016;6:4711–4730. doi: 10.1002/ece3.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burmester T. Evolution and function of the insect hexamerins. Eur J Entomol. 1999;96:213–226. [Google Scholar]
- 10.Busjahn A. wrappedtools: usefull wrappers around commonly used functions. R package version 0.3.10. 2020.
- 11.Castella G, Chapuisat M, Christe P. Prophylaxis with resin in wood ants. Anim Behav. 2008;75:1591–1596. doi: 10.1016/j.anbehav.2007.10.014. [DOI] [Google Scholar]
- 12.Cochran D. Nitrogen excretion in cockroaches. Annu Rev Entomol. 1985;30:29–49. doi: 10.1146/annurev.en.30.010185.000333. [DOI] [Google Scholar]
- 13.Cook SC, Eubanks MD, Gold RE, Behmer ST. Colony-level macronutrient regulation in ants: mechanisms, hoarding and associated costs. Anim Behav. 2010;79:429–437. doi: 10.1016/j.anbehav.2009.11.022. [DOI] [Google Scholar]
- 14.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 15.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 16.Cremer S, Armitage SAO, Schmid-Hempel P. Social immunity. Curr Biol. 2007;17:R693–R702. doi: 10.1016/j.cub.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Davis HE, Meconcelli S, Radek R, McMahon DP. Termites shape their collective behavioural response based on stage of infection. Sci Rep. 2018;8:14433. doi: 10.1038/s41598-018-32721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Roode JC, Lefevre T, Hunter MD. Self-medication in animals. Science. 2013;340:150–151. doi: 10.1126/science.1235824. [DOI] [PubMed] [Google Scholar]
- 19.Dussutour A, Simpson SJ. Communal nutrition in ants. Curr Biol. 2009;19:740–744. doi: 10.1016/j.cub.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Fanson BG, Weldon CW, Pérez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 21.Færgeman NJ, Wadum M, Feddersen S, Burton M, Kragelund BB, Knudsen J. Acyl-CoA binding proteins; structural and functional conservation over 2000 MYA. Mol Cellul Biochem. 2007;299:55–65. doi: 10.1007/s11010-005-9040-3. [DOI] [PubMed] [Google Scholar]
- 22.Felton GW, Summers CB. Antioxidant systems in insects. Arch Ins Biochem. 1995;29:187–197. doi: 10.1002/arch.940290208. [DOI] [PubMed] [Google Scholar]
- 23.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Method Softw. 2012;27:233–249. doi: 10.1080/10556788.2011.597854. [DOI] [Google Scholar]
- 24.Fujisawa K, Murakami R, Horiguchi T, Noma T. Adenylate kinase isozyme 2 is essential for growth and development of Drosophila melanogaster. Comp Biochem Physiol. 2009;153:29–38. doi: 10.1016/j.cbpb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Graham RI, Deacutis JM, Pulpitel T, Ponton F, Simpson SJ, Wilson K. Locusts increase carbohydrate consumption to protect against a fungal biopesticide. J Insect Physiol. 2014;69:27–34. doi: 10.1016/j.jinsphys.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haine ER, Rolff J, Siva-Jothy MT. Functional consequences of blood clotting in insects. Dev Comp Immunol. 2007;31:456–464. doi: 10.1016/j.dci.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton RL, Schal C. Effects of dietary protein levels on reproduction and food consumption in the German cockroach (Dictyoptera: Blattellidae) Ann Entomol Soc Am. 1988;81:969–976. doi: 10.1093/aesa/81.6.969. [DOI] [Google Scholar]
- 29.Hamilton RL, Cooper RA, Schal C. The influence of nymphal and adult dietary protein on food intake and reproduction in female brown-banded cockroaches. Entomol Exp Appl. 1990;55:23–31. doi: 10.1111/j.1570-7458.1990.tb01344.x. [DOI] [Google Scholar]
- 30.Hart BL. Behavioural defences in animals against pathogens and parasites: Parallels with the pillars of medicine in humans. Philos T R Soc B. 2011;366:3406–3417. doi: 10.1098/rstb.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He S, Johnston PR, Kuropka B, Lokatis S, Weise C, Plarre R, Kunte HJ, McMahon DP. Termite soldiers contribute to social immunity by synthesizing potent oral secretions. Insect Mol Biol. 2018;27:564–576. doi: 10.1111/imb.12499. [DOI] [PubMed] [Google Scholar]
- 32.He S, Sieksmeyer T, Che Y, Esparza-Mora MMA, Stiblik P, Banasiak R, Harrison MC, Šobotník J, Wang Z, Johnston PR, McMahon DP. Evidence for reduced immune gene diversity and activity during the evolution of termites. Proc R Soc B. 2021;288:20203168. doi: 10.1098/rspb.2020.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann F, Maser E. Carbonyl reductases and pluripotent hydroxysteroid dehydrogenases of the short-chain dehydrogenase/reductase superfamily. Drug Metab Rev. 2007;39:87–144. doi: 10.1080/03602530600969440. [DOI] [PubMed] [Google Scholar]
- 34.Hothorn T, Bretz F, Westfall P, Heiberger R. Multcomp: simultaneous inference for general linear hypotheses. R Package Version 1.0-3. 2008.
- 35.Jensen K, Silverman J. Frequently mated males have higher protein preference in German cockroaches. Behav Ecol. 2018;29:1453–1461. [Google Scholar]
- 36.Jensen K, McClure C, Priest NK, Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell. 2015;14:605–615. doi: 10.1111/acel.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen K, Schal C, Silverman J. Adaptive contraction of diet breadth affects sexual maturation and specific nutrient consumption in an extreme generalist omnivore. J Evol Biol. 2015;28:906–916. doi: 10.1111/jeb.12617. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RW, Curtis SE, Dantzer R, Bahr JM, Kelley KW. Sickness behavior in birds caused by peripheral or central injection of endotoxin. Physiol Behav. 1993;53:343–348. doi: 10.1016/0031-9384(93)90215-2. [DOI] [PubMed] [Google Scholar]
- 39.Jomori T, Natori S. Molecular-cloning of cDNA for lipopolysaccharide-binding protein from the hemolymph of the American cockroach, Periplaneta americana—similarity of the protein with animal lectins and its acute phase expression. J Biol Chem. 1991;266:13318–13323. doi: 10.1016/S0021-9258(18)98841-1. [DOI] [PubMed] [Google Scholar]
- 40.Kacsoh BZ, Lynch ZR, Mortimer NT, Schlenke TA. Fruit flies medicate offspring after seeing parasites. Science. 2013;339:947–950. doi: 10.1126/science.1229625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassambara A, Kosinski M, Biecek P. survminer: drawing survival curves using “ggplot2”. R package version 0.4.8. 2020.
- 42.Kassambara A. Rstatix: Pipe-friendly framework for basic statistical tests. R package version 0.6.0. 2020.
- 43.Kluger MJ, Rothenburg BA. Fever and reduced iron - their interaction as a host defense response to bacterial-infection. Science. 1979;203:374–376. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- 44.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/S0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc R Soc Ser B-Bio. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefevre T, Chiang A, Kelavkar M, Li H, Li J, de Castillejo CLF, Oliver L, Potini Y, Hunter MD, de Roode JC. Behavioural resistance against a protozoan parasite in the monarch butterfly. J Anim Ecol. 2012;81:70–79. doi: 10.1111/j.1365-2656.2011.01901.x. [DOI] [PubMed] [Google Scholar]
- 48.Lefevre T, Oliver L, Hunter MD, de Roode JC. Evidence for trans-generational medication in nature. Ecol Lett. 2010;13:1485–1493. doi: 10.1111/j.1461-0248.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 49.Li S, Zhu S, Jia Q, Yuan D, Ren C, Li K, Liu S, Cui Y, Zhao H, Cao Y, Fang G, Li D, Zhao X, Zhang J, Yue Q, Fan Y, Yu X, Feng Q, Zhan S. The genomic and functional landscapes of developmental plasticity in the American cockroach. Nat Comm. 2018;9:1008. doi: 10.1038/s41467-018-03281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason PA, Smilanich AM, Singer MS. Reduced consumption of protein-rich foods follows immune challenge in a polyphagous caterpillar. J Exp Biol. 2014;217:2250–2260. doi: 10.1242/jeb.093716. [DOI] [PubMed] [Google Scholar]
- 51.Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 52.McMahon DP, Hayward A. Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecol Entomol. 2016;41:505–515. doi: 10.1111/een.12313. [DOI] [Google Scholar]
- 53.Milan NF, Kacsoh BZ, Schlenke TA. Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Curr Biol. 2012;22:488–493. doi: 10.1016/j.cub.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohamed MA. Purification and characterization of alpha-amylase from the infective juveniles of the nematode Heterorhabditis bacteriophora. Comp Biochem Physiol. 2004;139:1–9. doi: 10.1016/j.cbpc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Moore J. An overview of parasite-induced behavioral alterations—and some lessons from bats. J Exp Biol. 2013;216:11–17. doi: 10.1242/jeb.074088. [DOI] [PubMed] [Google Scholar]
- 56.Natochin YuV, Parnova RG. Osmolality and electrolyte concentration of hemolymph and the problem of ion and volume regulation of cells in higher insects. Comp Biochem Physiol. 1987;88A:563–570. doi: 10.1016/0300-9629(87)90082-X. [DOI] [Google Scholar]
- 57.Pasco MY, Léopold P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS ONE. 2012;7:e36583. doi: 10.1371/journal.pone.0036583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patiño-Navarrete R, Piulachs M-D, Belles X, Moya A, Latorre A, Peretó J. The cockroach Blattella germanica obtains nitrogen from uric acid through a metabolic pathway shared with its bacterial endosymbiont. Biol Lett. 2014;10:20140407. doi: 10.1098/rsbl.2014.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pirk CW, Boodhoo C, Human H, Nicolson SW. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata) Apidologie. 2010;41:62–72. doi: 10.1051/apido/2009055. [DOI] [Google Scholar]
- 60.Pomés A, Wunschmann S, Hindley J, Vailes L, Chapman M. Cockroach allergens: function, structure and allergenicity. Protein Peptide Lett. 2007;14:960–969. doi: 10.2174/092986607782541178. [DOI] [PubMed] [Google Scholar]
- 61.Ponton F, Lalubin F, Fromont C, Wilson K, Behm C, Simpson SJ. Hosts use altered macronutrient intake to circumvent parasite-induced reduction in fecundity. Int J Parasitol. 2011;41:43–50. doi: 10.1016/j.ijpara.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Povey S, Cotter SC, Simpson SJ, Lee KP, Wilson K. Can the protein costs of bacterial resistance be offset by altered feeding behaviour? J Anim Ecol. 2009;78:437–446. doi: 10.1111/j.1365-2656.2008.01499.x. [DOI] [PubMed] [Google Scholar]
- 63.Povey S, Cotter SC, Simpson SJ, Wilson K. Dynamics of macronutrient self-medication and illness-induced anorexia in virally infected insects. J Anim Ecol. 2013;83:245–255. doi: 10.1111/1365-2656.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.R Core Team. R: a Language and Environment for Statistical Computing. 2020.
- 65.Ragheb R, Chuyen A, Torres M, Defaye A, Seyres D, Kremmer L, Fernandez-Nunez N, Tricoire H, Rihet P, Nguyen C, Roder L, Perrin L. Interplay between trauma and Pseudomonas entomophila infection in flies: a central role of the JNK pathway and of CrebA. Sci Rep. 2017;7:1. doi: 10.1038/s41598-017-14969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rapkin J, Jensen K, Archer CR, House CM, Sakaluk SK, Castillo ED, Hunt J. The geometry of nutrient space–based life-history trade-offs: sex-specific effects of macronutrient intake on the trade-off between encapsulation ability and reproductive effort in decorated crickets. Am Nat. 2018;191:452–474. doi: 10.1086/696147. [DOI] [PubMed] [Google Scholar]
- 67.Raubenheimer D, Jones SA. Nutritional imbalance in an extreme generalist omnivore: tolerance and recovery through complementary food selection. Anim Behav. 2006;71:1253–1262. doi: 10.1016/j.anbehav.2005.07.024. [DOI] [Google Scholar]
- 68.Reber A, Purcell J, Buechel SD, Buri P, Chapuisat M. The expression and impact of antifungal grooming in ants. J Evolution Biol. 2011;24:954–964. doi: 10.1111/j.1420-9101.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 69.Rivett AJ. Proteasomes: multicatalytic proteinase complexes. Biochemical J. 1993;291:1. doi: 10.1042/bj2910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA. Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften. 1999;86:544–548. doi: 10.1007/s001140050672. [DOI] [PubMed] [Google Scholar]
- 71.Rosengaus RB, Maxmen AB, Coates LE, Traniello JFA. Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae) Behav Ecol Sociobiol. 1998;44:125–134. doi: 10.1007/s002650050523. [DOI] [Google Scholar]
- 72.Sabree ZL, Kambhampati S, Moran NA. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc Natl Acad Sci. 2009;106:19521–19526. doi: 10.1073/pnas.0907504106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid-Hempel P. Parasites in social insects. Princeton: Princeton University Press; 1998. [Google Scholar]
- 74.Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc R Soc Ser B-Bio. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sehnal F, Svacha P, Zrzavy J. Evolution of insect metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis: Postembryonic reprogramming of gene expression in amphibian and insect cells. Academic Press; 1996. pp. 3–58. [Google Scholar]
- 76.Shik JZ, Schal C, Silverman J. Diet specialization in an extreme omnivore: nutritional regulation in glucose-averse German cockroaches. J Evol Biol. 2014;27:2096–2105. doi: 10.1111/jeb.12458. [DOI] [PubMed] [Google Scholar]
- 77.Simone M, Evans JD, Spivak M. Resin collection and social immunity in honey bees. Evolution. 2009;63:3016–3022. doi: 10.1111/j.1558-5646.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 78.Simpson SJ, Clissold FJ, Lihoreau M, Ponton F, Wilder SM, Raubenheimer D. Recent advances in the integrative nutrition of arthropods. Ann Rev Entomol. 2015;60:293–311. doi: 10.1146/annurev-ento-010814-020917. [DOI] [PubMed] [Google Scholar]
- 79.Simpson SJ, Raubenheimer D. Macronutrient balance and lifespan. Aging-Us. 2009;1:875–880. doi: 10.18632/aging.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singer MS, Carriere Y, Theuring C, Hartmann T. Disentangling food quality from resistance against parasitoids: diet choice by a generalist caterpillar. Am Nat. 2004;164:423–429. doi: 10.1086/423152. [DOI] [PubMed] [Google Scholar]
- 81.Singer MS, Mace KC, Bernays EA. Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars. PLoS ONE. 2009;4:e4796. doi: 10.1371/journal.pone.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siva-Jothy MT, Moret Y, Rolff J. Insect immunity: an evolutionary ecology perspective. Adv Insect Physiol. 2005;32:1–48. doi: 10.1016/S0065-2806(05)32001-7. [DOI] [Google Scholar]
- 83.Skaug H, Fournier D, Bolker B, Magnusson A, Nielsen A. Generalized linear mixed models using AD Model Builder. R package version 0.8. 0. 2014.
- 84.Smartt CT, Chiles J, Lowenberger C, Christensen BM. Biochemical analysis of a blood meal-induced Aedes aegypti glutamine synthetase gene. Insect Biochem Molec. 1998;28:935–945. doi: 10.1016/S0965-1748(98)00073-3. [DOI] [PubMed] [Google Scholar]
- 85.Smilanich AM, Mason PA, Sprung L, Chase TR, Singer MS. Complex effects of parasitoids on pharmacophagy and diet choice of a polyphagous caterpillar. Oecologia. 2011;165:995–1005. doi: 10.1007/s00442-010-1803-1. [DOI] [PubMed] [Google Scholar]
- 86.Sprouffske K. Growthcurver: simple metrics to summarize growth curves. R package version 0.3.1. 2020.
- 87.Srygley RB, Jaronski ST. Protein deficiency lowers resistance of Mormon crickets to the pathogenic fungus Beauveria bassiana. J Insect Physiol. 2018;105:40–45. doi: 10.1016/j.jinsphys.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Srygley RB. Diet drives the collective migrations and affects the immunity of Mormon crickets and locusts: a comparison of these potential superspreaders of disease. Integr Comp Biol. 2016;56:268–277. doi: 10.1093/icb/icw035. [DOI] [PubMed] [Google Scholar]
- 89.Srygley RB. Mormon crickets maximize nutrient intake at the expense of immunity. Physiol Entomol. 2017;42:1–9. doi: 10.1111/phen.12155. [DOI] [Google Scholar]
- 90.Terra WR, Ferreira C. Insect digestive enzymes: properties, compartmentalization and function. Comp Biochem Physiol. 1994;109:1–62. doi: 10.1016/0300-9629(94)90307-7. [DOI] [Google Scholar]
- 91.Therneau T. coxme: mixed effects Cox models. R package version 2.2–16. 2020.
- 92.Tragust S, Ugelvig LV, Chapuisat M, Heinze J, Cremer S. Pupal cocoons affect sanitary brood care and limit fungal infections in ant colonies. BMC Evol Biol. 2013;13:225. doi: 10.1186/1471-2148-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tyanova S, Temu T, Carlson A, Sinitcyn P, Mann M, Cox J. Visualization of LC-MS/MS proteomics data in MaxQuant. Proteomics. 2015;15:1453–1456. doi: 10.1002/pmic.201400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vallet-Gely I, Novikov A, Augusto L, Liehl P, Bolbach G, Pechy-Tarr M, Cosson P, Keel C, Caroff M, Lemaitre B. Association of hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, with cyclic lipopeptide production. Appl Environ Microb. 2010;76:910–921. doi: 10.1128/AEM.02112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Niekerk G, Isaacs AW, Nell T, Engelbrecht AM. Sickness-associated anorexia: mother nature’s idea of immunonutrition? Mediat Inflamm. 2016;2016:8071539. doi: 10.1155/2016/8071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Niekerk G, Loos B, Nell T, Engelbrecht AM. Autophagy - a free meal in sickness-associated anorexia. Autophagy. 2016;12:727–734. doi: 10.1080/15548627.2016.1147672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson K, Holdbrook R, Reavey CE, Randall JL, Tummala Y, Ponton F, Simpson SJ, Smith JA, Cotter SC. Osmolality as a novel mechanism explaining diet effects on the outcome of infection with a blood parasite. Curr Biol. 2020;30:2459–2467. doi: 10.1016/j.cub.2020.04.058. [DOI] [PubMed] [Google Scholar]
- 98.Wong AC, Holmes A, Ponton F, Lihoreau M, Wilson K, Raubenheimer D, Simpson SJ. Behavioral microbiomics: a multi-dimensional approach to microbial influence on behavior. Front Microbiol. 2015;6:1359. doi: 10.3389/fmicb.2015.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yanagawa A, Fujiwara-Tsujii N, Akino T, Yoshimura T, Yanagawa T, Shimizu S. Behavioral changes in the termite, Coptotermes formosanus (Isoptera), inoculated with six fungal isolates. J Invertebr Pathol. 2011;107:100–106. doi: 10.1016/j.jip.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Yanagawa H-A. Tissue distribution, purifications, and properties of multiple forms of hexokinase in the silkworm, Bombyx mori. Insect Biochem. 1978;8:293–305. doi: 10.1016/0020-1790(78)90040-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Protein database used for the proteomic analyses of the cockroach hemolymph.
Additional file 2. Data, diet choice following sublethal infection experiment.
Additional file 3. Data, survival on enforced diet experiment.
Additional file 4. Data, survival on enforced diet experiment after infection.
Additional file 5. Data, survival on enforced diet experiment after infection, plus deaths.
Additional file 6. Data, bacterial growth inhibition experiment.
Additional file 7. Results of the growthcruver analysis.
Additional file 8. Data, bacterial growth inhibition experiment (combined dataset) (Supplementary Fig. 3).
Additional file 9. Results of the growthcruver analysis (combined dataset).
Additional file 13. Supplementary File (Supplementary Tables andFigures).
Data Availability Statement
The short read data used to generate the Blatta orientalis transcriptome are available on the SRA (ID: SRX8891863, part of Bioproject PRJNA635910). All other data generated or analyzed during this study are included in this published article and its additional information files.