Figure 3.
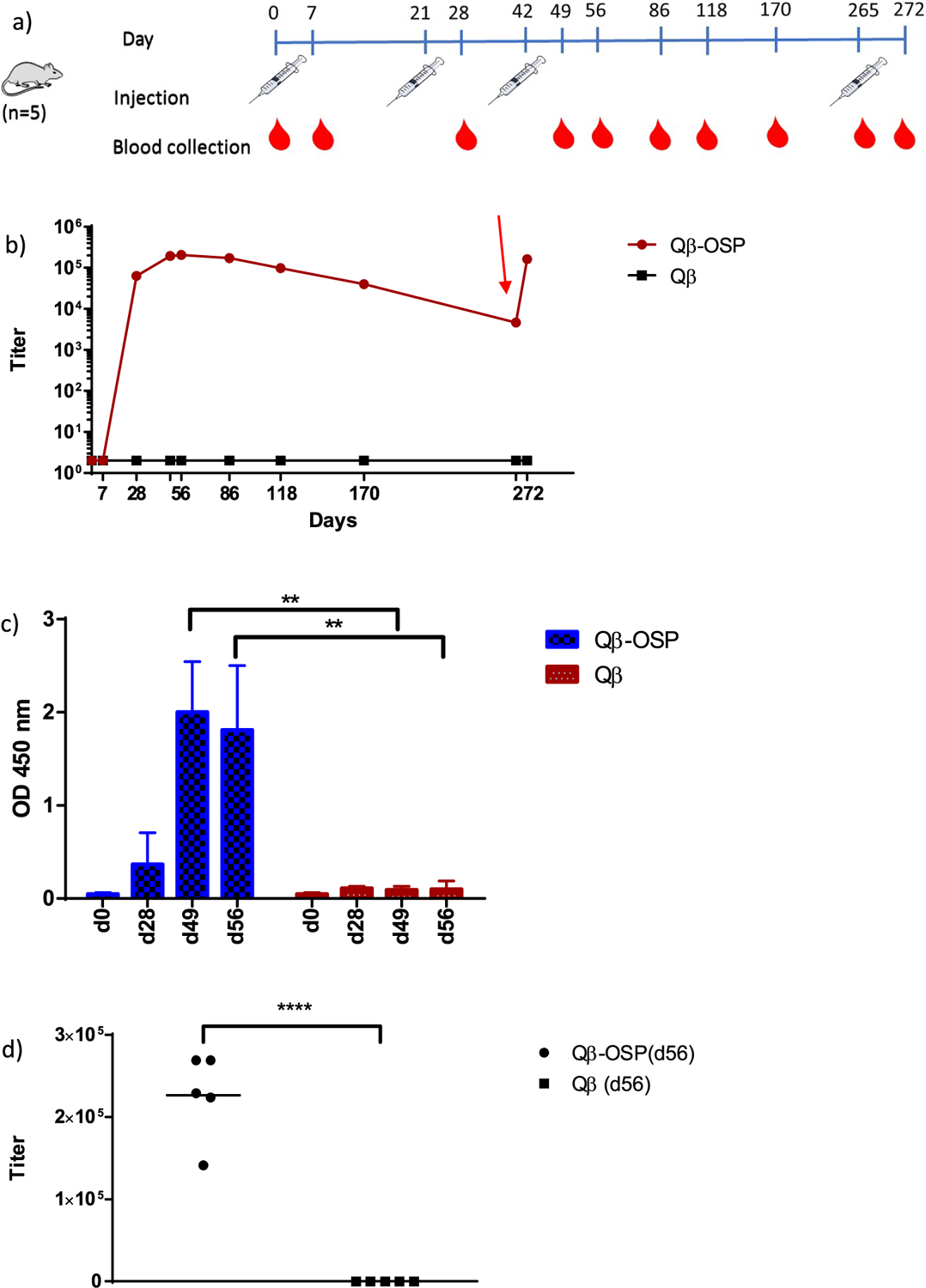
Evaluation of Qβ-OSP immunogenicity. (a) Immunization and blood collection schedule. Each group received 3 immunizations 3 weeks apart with blood collected at day 0 and on days 7, 28, 49, 56, 86, 118, 170, 265, and 272 respectively. (b) OSP-specific IgG titer of pooled sera from Qβ and Qβ-OSP groups up to day 272 postimmunization. The red arrow indicates a booster injection at day 265. (c) ELISA analysis showed significant IgG binding to BSA–OSP by postimmune sera at d49 and d56 (p = 0.0014 and 0.0065, respectively), compared to the control sera from mice immunized with Qβ only. Each bar represents data for 5 mice at 20 000 fold of serum dilution. (d) Individual mouse serum OSP-specific IgG titer of Qβ and Qβ-OSP groups at day 56. The statistical significance was determined through a two-tailed t test using GraphPad Prism. **p < 0.0001.
