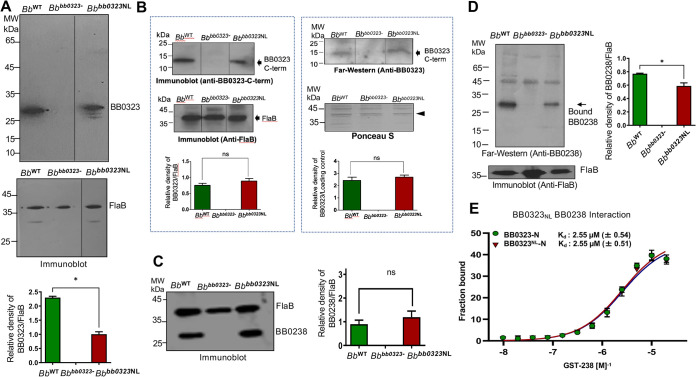
FIG 5.
The cleavage site mutant Bbbb0323NL isolates generate lesser amounts of functional N-terminal proteins, resulting in suboptimal recruitment of the interaction partner BB0238. (A) The Bbbb0323NL cleavage site mutant isolates produce significantly lower levels of mature 27-kDa N-terminal BB0323 protein. Comparable amounts of spirochete lysates from the WT, bb0323 deletion mutant (bb0323-), and the cleavage site mutant (Bbbb0323NL) isolates were separated by SDS-PAGE. The gel was then transferred to a nitrocellulose membrane and immunoblotted with antibodies against BB0323 (upper portion). The loading control was measured with anti-FlaB (lower portion). The densities of N-terminal protein were quantified by ImageJ software, with the values normalized against the corresponding density of FlaB. The amount of the upper band of BB0323NL protein was significantly decreased compared to the wild type (*, P < 0.05). (B) Analysis of C-terminal BB0323 fragments in Bbbb0323NL isolates. The Bbbb0323NL point mutation did not affect cleavage of the BB0323 C terminus (arrow, left portion). The proteins of WT, bb0323 mutant (bb0323), and Bbbb0323NL complemented spirochetes were separated using SDS-PAGE and transferred to a nitrocellulose membrane, which was probed with antibodies against the BB0323 C terminus (left upper portion), while the equal protein loading was shown by immunoblotting with anti-FlaB antibodies (arrow, left lower portion). The BB0323 C-terminal protein binds to the N-terminal BB0323, as assessed by far-Western analysis (arrow, right upper portion). The proteins of WT, bb0323 mutant (bb0323-), and Bbbb0323NL complemented spirochetes were separated using SDS-PAGE and transferred to a nitrocellulose membrane, which was incubated with recombinant BB0323-N protein purified from E. coli and probed with antiserum against the N terminus of BB0323 (right upper portion), while the equal protein loading is shown by Ponceau S stain (right lower portion). The bottom graphs represent the densities of the protein bands of corresponding BB0323 immunoblots, normalized against controls, either FlaB (left portion) or a protein stained with Ponceau S (arrowhead, right portion). (C) The cleavage site mutation did not reduce BB0238 protein levels in Bbbb0323NL. The protein lysates of WT, bb0323 mutant (bb0323-), and Bbbb0323NL isolates were separated by SDS-PAGE. The BB0238 protein level was detected with specific antibodies against BB0238 using Western blotting, with similar levels observed in Bbbb0323NL and the wild type. (D) Only mature 27-kDa N-terminal BB0323 protein is capable of interaction with BB0238. The protein lysates of WT, bb0323-, and Bbbb0323NL isolates were separated by SDS-PAGE and transferred to a nitrocellulose membrane, incubated with BB0238 recombinant protein, and probed with specific anti-BB0238 (upper portion). Only the mature 27-kDa N-terminal BB0323 peptides, not the lower BB0323 polypeptides, displayed binding. The density of the protein bands was scanned and normalized with FlaB (lower portion). The level of recruited BB0238 in the protein complex was significantly decreased compared to the wild-type protein (*, P < 0.05). (E) The binding affinity of recombinant BB0323 N-terminal proteins with BB0238 is not altered by cleavage site mutation. A microscale thermophoresis assay was performed to measure binding kinetics of BB0323-N and BB0323NL-N, which were essentially similar.