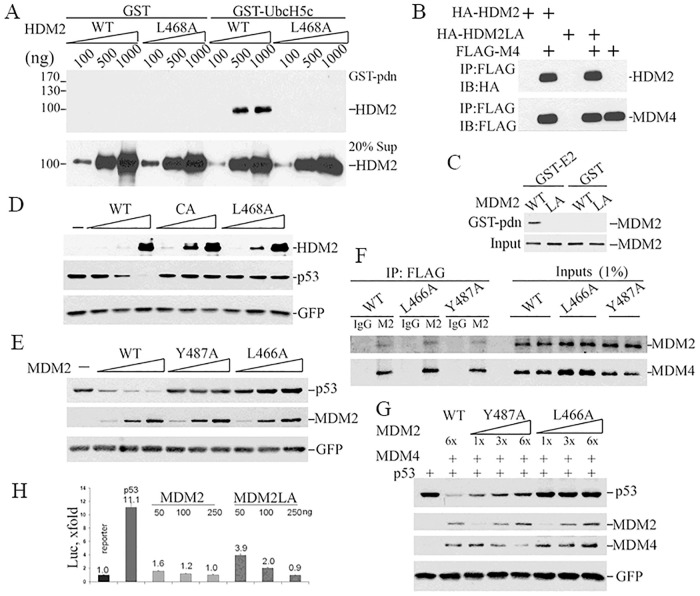
Fig 1. Effects of the L468/466A mutation on MDM2 protein activity.
(A) Increasing amounts of wild type or L468A HDM2) were mixed with GST-UbcH5c, or GST as a negative control, and physical interaction measured by pulldown assay. GST bound proteins (upper panel), or protein input (lower panel), were analyzed by western blotting using an HDM2 specific antibody. The position of protein molecular mass markers is listed at left. (B) Interaction between human recombinant FLAG-MDM4 (FLAG-M4) and HA-HDM2 (WT) or HA-HDM2L468A (HA-HDM2LA) in vitro was measured by pulldown assay using anti-FLAG M2 beads followed by WB for HA (HDM2, upper panel) or FLAG (HDM4, lower panel). (C) E2 binding activity was assessed using cytosolic proteins from 293T cells transfected with plasmids expressing MDM2 or MDM2L466A. Extracts were incubated with GST-UbcH5c or GST in pulldown assays. E2-bound MDM2 was detected by IB with 2A10 MDM2 antibody. (D) To test whether HDM2L468A or HDM2C462A promotes p53 degradation, pCMV-hp53 (5ng) was co-transfected with 200ng, 500ng, 1000 ng DNA expressing HDM2 (WT) or HDM2C464A (CA) or HDM2L6468A (L468A) and 50ng pEGFP in p53/Mdm2 double knockout MEF. Cell lysates were collected 24 hours after transfection and subjected to IB for p53 (DO-1) and HDM2 (2A9+4B11) and GFP. (E) To test if MDM2L466A promotes p53 degradation, co-transfections were done as in C but with 15ng pCMV-hp53 and 200ng, 600ng, 1200ng DNA expressing MDM2 (WT) or MDM2Y487A (Y487A) or MDM2L466A (L466A) transfected into PC3 cells. (F) Co-immunoprecipitation was performed with cell lysates from p53/Mdm2/Mdm4 triple knockout MEFs co-transfected MDM4 with MDM2 (WT), MDM2Y487A (Y487A) or MDM2L466A(L466A). M2, anti-FLAG beads, or IgG as control antibody, were used to immunoprecipitate proteins. Antibodies 2A10 and rabbit 17914-1-AP were used to detect MDM2 and MDM4, respectively, on western blots. (G) Co-transfection was done similarly as in E with the exception of 600ng MDM4 was co-transfected with different versions of MDM2. (H) Inhibition of p53-dependent transcription by MDM2 or MDM2L466A (MDM2LA) in p53-/-/Mdm2-/- MEFs was analyzed using a luciferase reporter assay. p53 transcriptional activity is presented as fold increase against luciferase activity of the sample transfected with reporter plasmid alone.