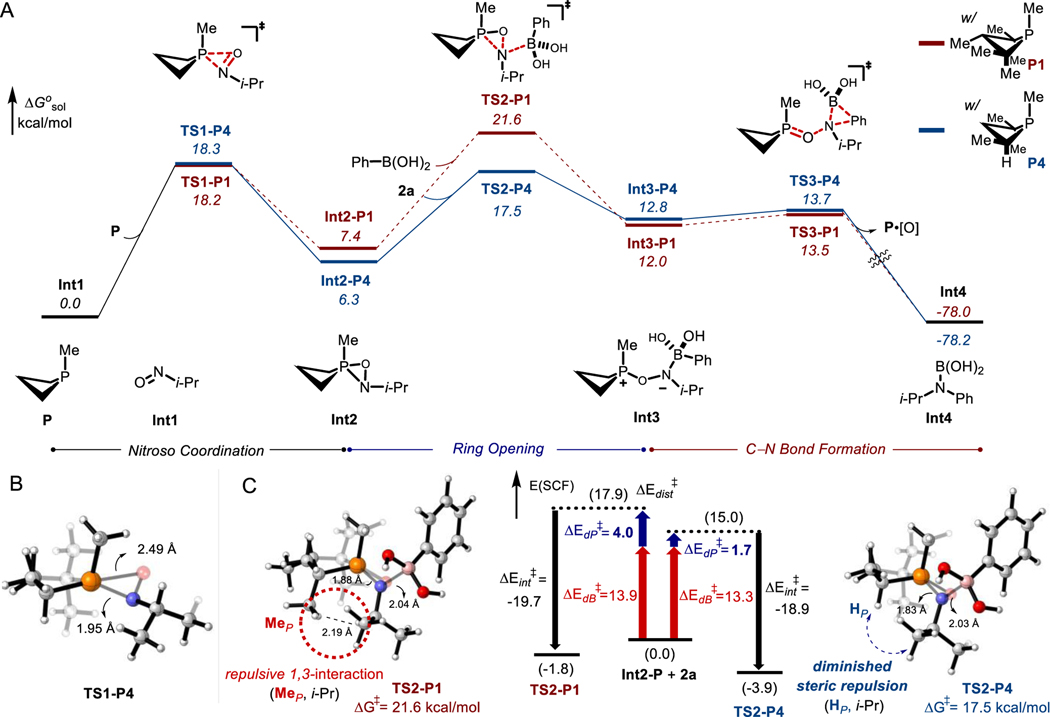
Figure 5.
(A) Mechanistic proposal for second deoxygenation and C–N bond formation supported by density functional theory (DFT) calculations at the PCM(PivCN)-B3LYP-D3/6–311++g(d,p)//6–31g(d,p) level of theory. Relative free energies (italics) are given in kcal/mol. (B) Computed model of TS1-P4. (C) Transition structures and distortion/interaction analyses for TS2-P: Oxazaphosphirane distortion energy (ΔEdP⧧) in blue, phenylboronic acid distortion energy (ΔEdB⧧) in red, fragment interaction energy (ΔEint⧧) in black.