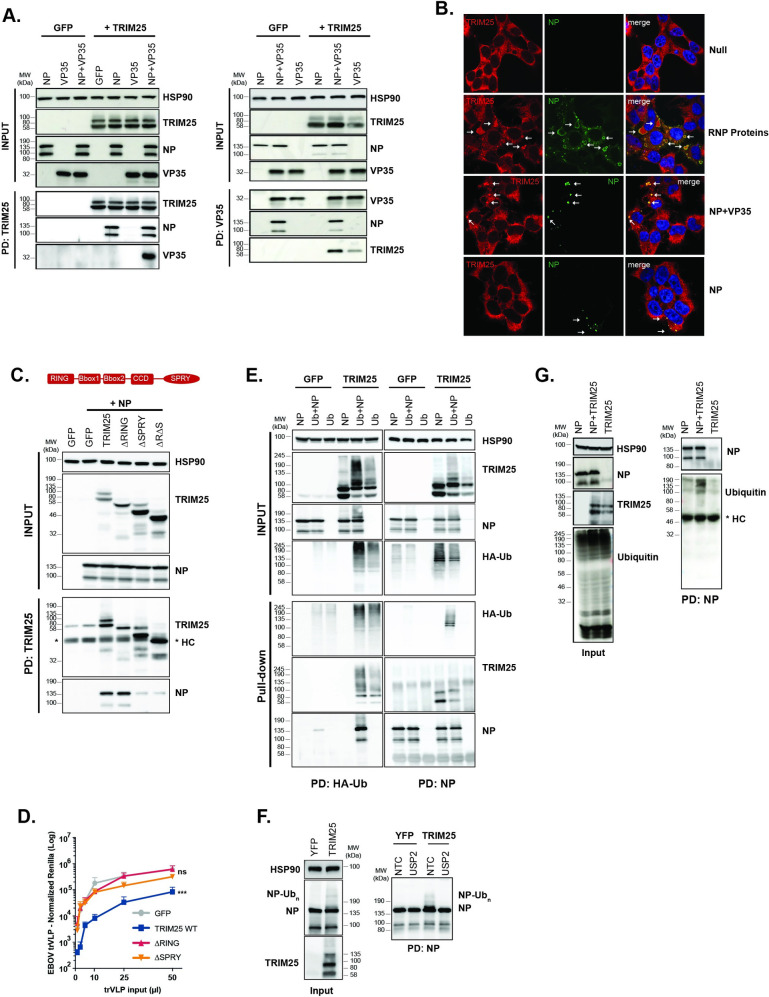
Fig 5. TRIM25 interacts with EBOV NP and promotes its ubiquitination.
(A) Lysates of HEK293T-TIM1 cells transfected either with GFP or TRIM25, in combination with EBOV NP and/or EBOV VP35, were immunoprecipitated with an anti-TRIM25 (left panel) or an anti-VP35 (right panel) antibodies. Cellular lysates and pull-downs were analysed by western blot for HSP90, TRIM25, EBOV NP and VP35. (B) Panels show representative fields for the localization of EBOV NP and endogenous TRIM25 on HEK293T-TIM1 cells left untreated (Null), or transfected with EBOV NP protein alone, or in combination either with VP35 or all remaining RNP proteins (VP35, VP30 and L). Cells were stained 24 hours post-transfection with anti-TRIM25 (red) and anti-NP (green) antibodies, as well as with DAPI (blue). White arrows point to the localization of TRIM25 intracellular aggregates. (C) Schematic representation of functional domains within TRIM25 (upper panel). Lysates of HEK293T-TIM1 cells transfected with EBOV NP in combination with GFP, TRIM25 or mutants thereof, were immunoprecipitated with an anti-TRIM25 antibody. Input and pull-down samples were blotted for HSP90, TRIM25 and EBOV NP (lower panel). (*) indicates the detected heavy-chains (HC) from the antibody used in the pull-down. (D) EBOV trVLP normalized reporter activity on HEK293T-TIM1 cells transfected with EBOV RNP proteins in combination with GFP (grey), TRIM25 wild-type (blue), TRIM25 ΔRING (red) or TRIM25 ΔSPRY (orange) mutants, prior to infection with increasing amounts of EBOV trVLPs (p1 target cells). EBOV trVLP Rluc reporter activities were measure 24 hours post-infection and normalized to control Fluc values obtained in the same lysates. EBOV trVLP Renilla reporter activities are normalized to control Firefly luciferase values obtained in the same lysates. *p > 0.05, **p > 0.01 and ***p > 0.001 as determined by two-tailed paired t-test. All error bars represent ± SEM of at least three independent experiments. (E) HEK293T-TIM1 cells were transfected either with GFP or TRIM25, in combination with EBOV NP and/or a plasmid expressing a HA-tagged Ubiquitin (HA-Ub). Lysates from these cells were immunoprecipitated with an anti-HA antibody (left panels) or an anti-NP antibody (right panels). Cellular lysates and pull-down samples were analysed by western blot for HSP90, TRIM25, EBOV NP and HA (ubiquitin). (F) Lysates from HEK293T cells co-transfected with EBOV NP and YFP or TRIM25 were immunoprecipitated with an anti-NP antibody, and pulled-down fractions treated with USP2 deubiquitinase enzyme. Cellular lysates and pull-downs were analysed by western blot for HSP90, TRIM25 and EBOV NP. (G) HEK293T-TIM1 were transfected with EBOV NP and/or TRIM25 under endogenous Ubiquitin levels and treated with Bafilomycin A (100nM). Lysates from the cells were immunoprecipitated with an anti-EBOV antibody. Cellular lysates and pull-down samples were analysed by western blot for HSP90, TRIM25, EBOV NP and Ubiquitin.