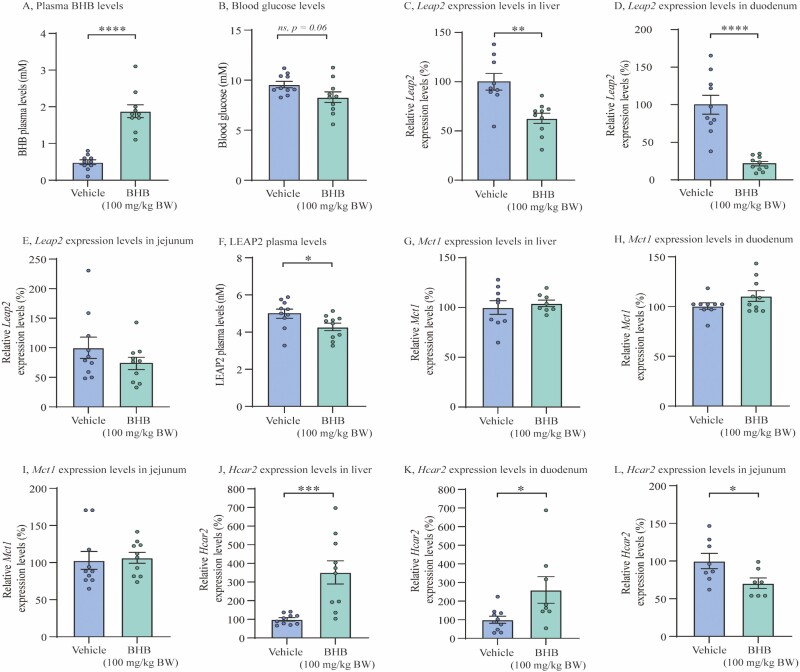
Figure 4.
In vivo administration of exogenous BHB downregulates circulating LEAP2 levels and Leap2 expression in mouse liver and duodenum. (A) Plasma levels of BHB in 2 treatment groups; vehicle and BHB (100 mg/kg BW), 2 hours after oral administration, (B) Blood glucose levels in 2 treatment groups; vehicle and BHB (100 mg/kg BW), 2 hours after oral administration, (C-E) Relative Leap2 expression levels in 2 treatment groups; vehicle and BHB (100 mg/kg BW), in liver, duodenal, and jejunal, respectively, 2 hours after oral administration, (F) Systemic LEAP2 plasma levels from vehicle and BHB (100 mg/kg BW), 2 hours after oral administration, (G-I) Relative Mct1 expression levels in 2 treatment groups; vehicle and BHB (100 mg/kg BW), in liver, duodenal, and jejunal, respectively, 2 hours after oral administration, (J-L) Relative Hcar2 expression levels in 2 treatment groups; vehicle and BHB (100 mg/kg BW), in liver, duodenum, and jejunum, 2 hours after oral administration. Data were normalized to reference gene Ywhaz and subsequently normalized to vehicle (C-E + G-L). All data were analyzed by unpaired t test. n = 10 in each group. Outliers were identified and exclusion of outlier did not diverge from the results of the main analysis and result (C, G, H, K, L). * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001, ns = not significant. Abbreviations: BHB; beta-hydroxybutyrate, Hcar2; hydroxycarboxylic acid receptor 2, LEAP2/Leap2; Liver-expressed antimicrobial peptide 2, Mct1; monocarboxylate transporter 1.