Abstract
Background
Fusobacterium nucleatum has been identified to promote tumor progression in colorectal cancer (CRC). However, association between F. nucleatum and prognostic or clinicopathological features has been diverse among studies, which could be affected by type of biospecimen (formalin-fixed paraffin-embedded or fresh frozen [FF]).
Methods
Articles were systemically reviewed for studies that included the correlation between F. nucleatum and prognosis or clinicopathological features in CRC.
Results
Ten articles, eight studies with survival-related features involving 3,199 patients and nine studies with clinical features involving 2,655 patients, were eligible for the meta-analysis. Overall survival, disease-free survival, and cancer-specific survival were all associated with worse prognosis in F. nucleatum–high patients (p<.05). In subgroup analysis, only studies with FF tissues retained prognostic significance with F. nucleatum. In meta-analysis of clinicopathological variables, F. nucleatum level was associated with location within colon, pT category, MLH1 hypermethylation, microsatellite instability status, and BRAF mutation regardless of type of biospecimen. However, lymph node metastasis and KRAS mutation was only associated with F. nucleatum level in FF-based studies.
Conclusions
In conclusion, type of biospecimen could affect the role of F. nucleatum as a biomarker associated with clinicopathological features and prognosis.
Keywords: Fusobacteria, Colorectal neoplasms, Prognosis
In recent years, it has been established that gut microbiota is closely linked to various intestinal human disease including colorectal cancer (CRC) via microbiome dysbiosis [1-3]. Among the heterogeneous microbiota, Fusobacterium nucleatum, an anaerobic, gram-negative bacteria, has been enriched in CRC tumor tissues and stool specimen compared with normal tissues [4,5]. Abundance of F. nucleatum is gradually increased from normal to precancerous adenomatous lesions to carcinoma in CRC and suggests a significant role of F. nucleatum in tumor progression of CRC [6,7].
F. nucleatum has various mechanisms that influence carcinogenesis which includes promoting of E-cadherin/β-catenin signaling via adhesion protein FadA [8], adhering to cancer cells, colonizing, and inhibiting immunity by leptin Fap2 [2,9], inducing development of CRC by mircoRNA-21–mediated pathway [10], and increasing chemoresistance via autophagy [11,12]. Recently, it has been reported that F. nucleatum is positive in CRC cells in liver metastases, which suggests that F. nucleatum may also act as an integral component in CRC metastasis [13]. Furthermore, in xenograft tumor model, treating F. nucleatum with antibiotics resulted in reduction of tumor growth.
Even though F. nucleatum plays a key role in CRC progression and metastasis, studies that report the characteristics of F. nucleatum in prognosis and clinicopatholigcal features by using CRC tissues, nevertheless, has shown conflicting results. Several studies considered F. nucleatum level as a suitable biomarker for worse survival [14-16] in CRC patients while other studies did not detect correlation between F. nucleatum level and prognosis in overall CRC patients [17,18]. Similar discrepancies between studies arise from other clinicopathological studies such as KRAS mutation, tumor location, and microsatellite instability (MSI) status. Although the reason for discordance could be due to heterogeneity in CRC patient populations [19], this could also be affected by the type of biospecimen used for each study, namely formalin-fixed paraffin-embedded (FFPE) tissue or fresh frozen (FF) tissue. A recent article addresses differences between paired FFPE and FF samples when detecting F. nucleatum in CRC [20], but a systemic review that discuss the impact of biospecimen in detecting F. nucleatum has not been available.
In this study, we conducted a systemic review and meta-analysis to determine the prognostic and clinicopathological significance of F. nucleatum in CRC. Our aim is to clarify how the selection of biospecimen could allocate different roles of microbiome of CRC by affecting the association between F. nucleatum level and survival or clinicopathological features.
MATERIALS AND METHODS
Literature search strategy
Articles relevant with the subject were retrieved from electronic databases, PubMed, Embase, and Web of Science until December 3 of 2020. Search terms were, “Fusobacterium nucleatum, Fusobacterium, Fusobacteria, or F. nucleatum” and “colorectal cancer, colon cancer, rectal cancer, colorectal carcinoma, or CRC”. Each article was examined carefully to determine studies with identical patient population. When multiple studies present overlapping patient data, we selected the studies with the largest patient cohort for clinical data and survival data, respectively.
Inclusion and exclusion criteria
Studies that were eligible for the inclusion in this meta-analysis were as follows: (1) full length articles published in English that detected Fusobacterium nucleatum from human tumor samples derived from CRC tissue, (2) studies with CRC samples divided into high and low (or high and low/negative) according to F. nucleatum level, (3) studies that included clinicopathological, molecular, or survival-associated data that were correlated with F. nucleatum level. Studies that only contain abstract or posters, studies could not be arranged as dichotomized F. nucleatum level data, studies with insufficient data, studies that used more than one type of biospecimen to detect F. nucleatum level, and studies that detected F. nucleatum level in stools were excluded.
Data extraction and quality assessment
Two reviewers (Y.K. and G.H.K.) independently collected data from eligible articles according to the criteria. Newcastle-Ottawa Scale was used to evaluate study quality. A score higher than six out of nine criteria was defined as qualified. Name of author, year of publication, number of total patients, number of F. nucleatum positive patients, type of biospeicmen (FFPE or FF), method of F. nucleatum level detection, survival data, and clinicopathological data associated with F. nucleatum level were collected from each study. Survival data were extracted as hazard ratio [HR] and 95% confidence intervals [CIs] from univariate Cox analysis. When only Kaplan-Meier curves were presented for survival analysis, HR and 95% CIs were estimated by using Engauge Digitizer V9.8 (http://digitizer.sourceforge.net) and spreadsheets provided by Tierney et al. [21]. When a three-tier survival was given (negative, low, and high) we pooled negative and low as reference to calculate HR and 95% CIs for high and rearranged it into two-tier (low/negative and high).
Statistical analysis
Meta-analysis was conducted using R software ver. 3.5.3 (https://cran.r-project.org/). Pooled HR and 95% CIs for overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS) and pooled odds ratio and 95% CIs for clinicopathological features were analyzed via R package ‘meta’ and ‘dmetar’. Subgroup analysis was performed if multiple types of biospecimen existed within a pooled analysis. Statistic heterogeneity was determined by Cochran’s Q and I2. When heterogeneity was observed (p<.05 or I2>50%) random effect model was used for the analysis instead of fixed effect model. A graphical funnel plot was used to evaluate publication bias.
RESULTS
Literature search and study characteristics
Four hundred and thirty-two records were collected from PubMed, Embase, and Web of Science (Fig. 1). After removing 76 duplications, 356 records were screened by abstract and 32 records were categorized as being relevant with the subject. These records were reviewed in detail and 10 articles, eight studies with survival-related features involving 3,199 patients [14-18,22-24] and nine studies with clinical features involving 2,655 patients [14-17,22-25], were selected eligible for the meta-analysis (Table 1). Seven studies were included in the meta-analysis for both survival-related and clinical features [14-17,22-24]. Two studies had overlapping patients but were used for meta-analysis of either clinical or survival-related features, respectively [18,25]. Six out of the 10 articles assessed F. nucleatum level with FFPE tissues [6,16-18,24,25] and four articles used FF tissues to detect F. nucleatum [14,15,22,23].
Fig. 1.
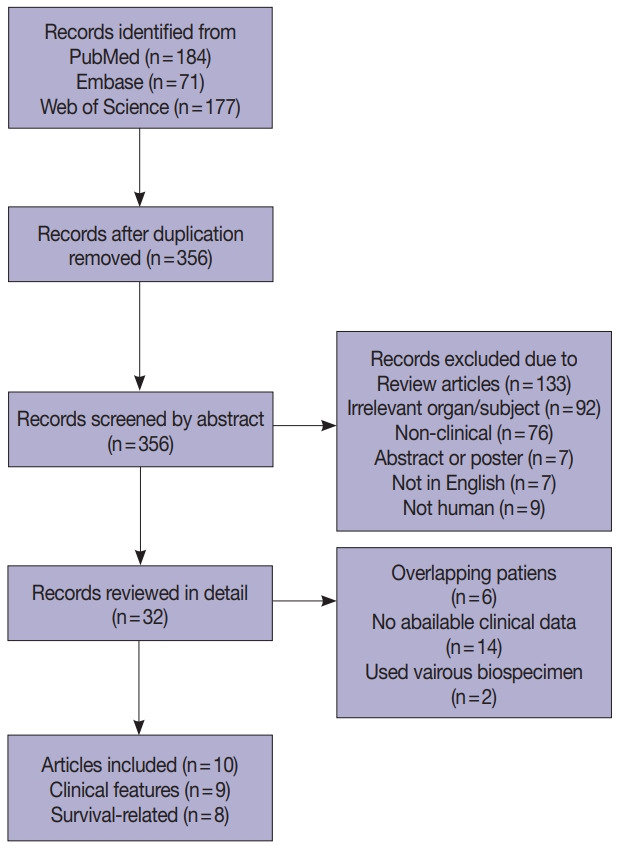
Flow chart of literature search and study selection.
Table 1.
Characteristics of articles included in the meta-analysis
| Authors | Year | No. of patients | No. of positives | Biospecimen | Method | Survival | Clinical | Stage |
|---|---|---|---|---|---|---|---|---|
| Yan et al. [16] | 2017 | 280 | 187 | FFPE | qPCR | O | O | III/IV |
| Wei et al. [14] | 2016 | 180 | 90 | FF | qPCR | O | O | I–III |
| Yamaoka et al. [15] | 2018 | 100 | 50 | FF | ddPCR | O | O | I–IV |
| Samkamoto et al. [18] | 2018 | 1,069 | 67 | FFPE | qPCR | O | I–IV | |
| Sun et al. [22] | 2016 | 152 | 59 | FF | qPCR | O | O | I–IV |
| Kunzmann et al. [23] | 2019 | 190 | 61 | FF | qPCR | O | O | I–IV |
| Oh et al. [17] | 2019 | 593 | 204 | FFPE | qPCR | O | O | II/III |
| Chen et al. [24] | 2019 | 91 | 25 | FFPE | qPCR | O | O | II/III |
| Ito et al. [6] | 2015 | 511 | 143 | FFPE | qPCR | O | I–IV | |
| Mima et al. [25] | 2015 | 1,102 | 69 | FFPE | qPCR | O | I–IV |
FFPE, formalin-fixed paraffin-embedded; qPCR, quantitative polymerase chain reaction; FF, fresh frozen; ddPCR, droplet digital polymerase chain reaction.
Meta-analysis between F. nucleatum level and survival
The meta-analysis between F. nucleatum level and prognostic values of OS (Fig. 2A), DFS (Fig. 2B), and CSS (Fig. 2C) in CRC has shown that high F. nucleatum level is associated with poor OS, DFS, and CSS (OS: HR, 1.58; 95% CI, 1.28 to 1.94; p<.001; DFS: HR, 1.76; 95% CI, 1.06 to 2.93; p=.030; CSS: HR, 1.72; 95% CI, 1.05 to 2.83; p=.031, respectively). In subgroup analysis, studies with FF samples (p<.001), but not those with FFPE samples (p=.168) retained prognostic significance between F. nucleatum level and OS (Table 2, Supplementary Fig. S1). Furthermore, studies with FFPE samples did not show significant correlation between F. nucleatum level and DFS (p=.214). Analyzing studies with stage I though IV CRC did not affect prognostic significance between F. nucleatum level and OS (HR, 1.41; 95% CI, 1.11 to 1.80; p=.005).
Fig. 2.
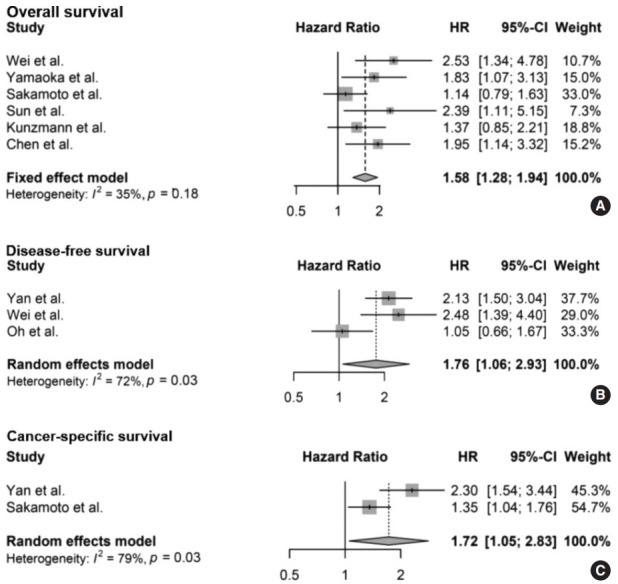
Forest plot for the correlation between Fusarium nucleatum and survival in colorectal cancer [14-18,22-24]: (A) overall survival, (B) disease-free survival, and (C) cancer-specific survival. HR, hazard ratio; CI, confidence interval.
Table 2.
Subgroup analysis associated with survival
| Survival | Specimen | Study | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Overall survival | FFPE | 2 | 1.43 | 0.85–2.42 | .168 |
| FF | 4 | 1.84 | 1.36–2.48 | < .001 | |
| Disease-free survival | FFPE | 2 | 1.52 | 0.76–3.04 | .214 |
HR, hazard ratio; CI, confidence interval; FFPE, formalin-fixed paraffin-embedded; FF, fresh frozen.
Meta-analysis between F. nucleatum level and clinicopathological features
Meta-analysis for correlation between F. nucleatum level and clinicopathological features of CRC are assessed in Table 3. High F. nucleatum level was associated with proximal colon location (p<.001), worse tumor differentiation (p=.002), high pT category (p<.001), presence of distal metastasis (p=.007), MLH1 hypermethylation (p<.001), and MSI-high CRC (p=.001) when all studies were considered. In subgroup analysis (Table 4), studies with FFPE samples showed significant correlation between high F. nucleatum level and proximal colon location (p=.001), worse tumor differentiation (p=.015), high pT category (p<.001), MLH1 hypermethylation (p<.001), CpG island methylator phenotype (CIMP)–high status (p<.001), and MSI-high status (p<.001). Studies with FF samples, on the other hand, demonstrated that high F. nucleatum level is significantly correlated with high pT category (p=.001), presence of lymph node metastasis (p=.001), and presence of KRAS mutation (p=.007), which showed borderline significance with high F. nucleatum level in overall studies (p=.052).
Table 3.
Meta-analysis associated with clinical features
| Clinical feature | Study | OR | 95% CI | p-value |
|---|---|---|---|---|
| Age | 5 | 0.993 | 0.787–1.254 | .954 |
| Sex (female/male) | 9 | 1.087 | 0.910–1.299 | .356 |
| Proximal/Distal colon | 4 | 1.642 | 1.252–2.153 | < .001 |
| Rectum/Colon | 5 | 1.184 | 0.899–1.560 | .229 |
| Tumor size | 2 | 0.897 | 0.592–1.360 | .609 |
| Differentiation | 7 | 1.547 | 1.166–2.052 | .002 |
| Vascular invasion | 2 | 0.627 | 0.337–1.167 | .141 |
| Perineural invasion (FFPE only) | 2 | 1.022 | 0.722–1.448 | .900 |
| pT category | 5 | 2.253 | 1.633–3.108 | < .001 |
| Lymph node metastasis | 5 | 1.308 | 0.979–1.748 | .069 |
| Distant metastasis | 4 | 1.912 | 1.196–3.058 | .007 |
| Stage | 6 | 1.110 | 0.885–1.394 | .367 |
| CIMP status (FFPE only) | 3 | 2.612 | 1.849–3.690 | < .001 |
| MLH1 hypermethylation | 3 | 2.852 | 1.987–4.095 | < .001 |
| MSI status | 5 | 2.982 | 2.175–4.086 | < .001 |
| KRAS mutation | 5 | 1.248 | 0.998–1.561 | .052 |
| BRAF mutation | 4 | 1.869 | 1.273–2.745 | .001 |
| PIK3CA mutation | 3 | 1.440 | 0.965–2.147 | .074 |
OR, odds ratio; CI, confidence interval; FFPE, formalin-fixed paraffin-embedded; CIMP, CpG island methylator phenotype; MSI, microsatellite instability.
Table 4.
Subgroup analysis for clinical features
| Clinical feature | Specimen | Study | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Age | FFPE | 2 | 1.158 | 0.871–1.539 | .312 |
| FF | 3 | 0.720 | 0.478–1.086 | .117 | |
| Sex | FFPE | 5 | 1.096 | 0.891–1.349 | .386 |
| FF | 4 | 1.063 | 0.753–1.501 | .727 | |
| Proximal/Distal colon | FFPE | 2 | 1.676 | 1.220–2.303 | .001 |
| FF | 2 | 1.554 | 0.925–2.612 | .096 | |
| Rectum/Colon | FFPE | 2 | 1.259 | 0.841–1.884 | .263 |
| FF | 3 | 1.120 | 0.767–1.636 | .557 | |
| Differentiation | FFPE | 4 | 1.513 | 1.083–2.112 | .015 |
| FF | 3 | 1.632 | 0.958–2.778 | .071 | |
| pT category | FFPE | 2 | 2.187 | 1.437–3.329 | < .001 |
| FF | 3 | 2.354 | 1.428–3.880 | .001 | |
| Lymph node metastasis | FFPE | 2 | 0.832 | 0.549–1.262 | .387 |
| FF | 3 | 2.009 | 1.337–3.017 | .001 | |
| Distant metastasis | FF | 3 | 1.669 | 0.863–3.228 | .128 |
| Stage | FFPE | 2 | 0.917 | 0.673–1.251 | .586 |
| FF | 4 | 1.389 | 0.993–1.943 | .055 | |
| MLH1 hypermethylation | FFPE | 2 | 3.210 | 2.208–4.667 | < .001 |
| MSI status | FFPE | 4 | 2.757 | 1.987–3.824 | < .001 |
| KRAS mutation | FFPE | 3 | 1.125 | 0.881–1.436 | .345 |
| FF | 2 | 2.220 | 1.246–3.954 | .007 | |
| BRAF mutation | FFPE | 3 | 1.882 | 1.260–2.811 | .002 |
| PIK3CA mutation | FFPE | 2 | 1.464 | 0.960–2.233 | .076 |
OR, odds ratio; CI, confidence interval; FFPE, formalin-fixed paraffin-embedded; FF, fresh frozen; MSI, microsatellite instability.
Publication bias
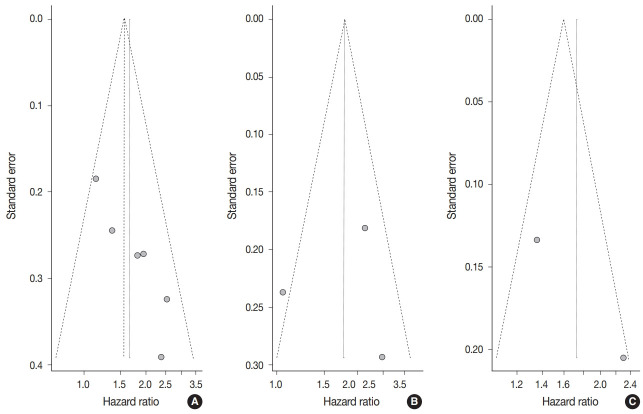
Begg’s funnel plot was evaluated for presence of potential publication bias in meta-analysis associated with survival (Fig. 3). Visible asymmetry was observed with Egger’s test reporting p-value of .006 for OS but not for DFS (p=.935).
Fig. 3.
Funnel plot for studies included in meta-analysis between Fusarium nucleatum level and survival: (A) overall survival, (B) disease-free survival, and (C) cancer-specific survival.
DISCUSSION
F. nucleatum has been associated with cancer development and progression of CRC, as well as a potential target of cancer treatment. F. nucleatum is abdudnant in CRC tissues from patients with metastasis than those from non-metastatic CRC patients [26]. Presence F. nucleatum was essential in establishment of CRC patients-derived xenografts in mice [13]. Not only does infection of F. nucleatum has promoted metastasis in CRC cells via autophage but also is found in distant metastasis sites including liver, lung, and lymph nodes, although the mechanism is yet to be unveiled [9,13,26,27]. Moreover, previous studies have suggested that F. nucleatum provoke chemoresistance inhibiting caspase cascade and activating autophagy pathway to prevent apoptosis in CRC cells [11,28].
It is still unclear if F. nucleatum is a significant driver of tumor initiation and F. nucleatum level detected from CRC tissues and association with prognosis and clinicopathological variables has not been consistent among studies. Herein, we hypothesized that this could be partially caused by different type of biospecimen being used for each study and investigated its affect by a systemic review and meta-analysis with the difference between FFPE and FF into consideration.
High F. nucleatum level was associated with worse survival in OS, DFS, and CSS in overall meta-analysis. Subgroup analysis revealed that studies with FF show a significant correlation between F. nucleatum level and OS. However, studies with FFPE tissues show that a significant correlation between F. nucleatum and OS or DFS is not observed.
Association between F. nucleatum level and clinicopathological features are much more diverse. A pooled analysis of FFPE and FF studies together shows that high F. nucleatum level samples are significantly enriched in proximal colon, but on subgroup analysis, only studies using FFPE samples retain statistical significance for the same variable. Similar findings could be observed for correlation between F. nucleatum level and distant metastasis. On the contrary, pT category, MLH1 hypermethylation, and MSI status remained its positive correlation with F. nucleatum level regardless of the biospecimen being used. Lymph node metastasis and KRAS mutation were only significant in FF tissues and MLH1 hypermethylation was only significant in FFPE tissues but not in its amalgamated state. Interestingly, the aforementioned features are all considered as hallmark characteristics of MSI-high CRC [29]. These findings emphasize that regardless of type of specimen, high F. nucleatum level is strongly indicative of MSI-high status.
There are several putative explanations for discrepancy of results between biospecimen. Mean patient number for studies with FFPE tissue (515.4 patients per study) is more than three times larger than the mean patient number for studies with FF tissues (155.5 patients per study). On the contrary, F. nucleatum positivity is significantly higher in studies with FF tissues compared with those of FFPE tissue (41.8% and 24.4%. respectively) which may be a result of a lower threshold in studies with FF tissues to compensate for smaller sample size. This may allude to the fact that features with relatively low incidence such as KRAS mutation, could have a higher chance of being categorized as high F. nucleatum level sample in studies with FF compared with studies with FFPE.
As an alternative explanation, lower F. nucleatum positivity in FFPE tissues may reflect the inherited nature of FFPE samples rather than inflation of threshold in studies with FF tissues. In a study that measured F. nucleatum level in paired FFPE and FF tissues of CRC, FF tissues had higher detection rate of overall F. nucleatum resulting in difference in survival and clinicopathological features from FFPE-measured samples [20]. Furthermore, concordance between F. nucleatum positive cases in FF and those in FFPE samples were low. Discrepancy between DNA results of FFPE and FF samples has also been reported in other paired tumor tissues [30]. This was in part due to inter-strand crosslink between complementary DNA strands which could be aggravated by prolonged fixation [31]. Other potential factors of low F. nucleatum detection in FFPE samples includes fragmentation and degeneration of DNA, paraffin embedding, and storage process [25,32].
Recently, two articles analyzed the correlations between F. nucleatum level and prognosis in CRC with meta-analysis [33,34]. Both articles showed a significant correlation between F. nucleatum level and OS, but only one study showed a significant correlation between F. nucleatum level and DFS. Herein, we suggest that type of biospecimen that was included in each meta-analysis could have affected the discrepancy between the articles.
Limitation of our study is that we cannot identify pathological and molecular discrepancies between FF and FFPE tissues such as correlation between F. nucleatum level and T cell density, CIMP, and various immunohistochemical results due to lack of studies with FF that obtain related data. Moreover, although metaanalysis provides evidence that discordance between FF and FFPE samples are present in detecting F. nucleatum, we could not narrow down the cause of the misalignment between the two biospecimen.
In conclusion, F. nucleatum level is significantly associated with pathological and molecular features including pT category, MLH1 hypermethylation, and MSI status regardless of biospecimen, but other survival, clinical, and molecular features, such as location of tumor within the colon, distant metastasis, and KRAS mutation, could be affected by type of biospecimen in CRC. Therefore, although a strong association between MSI and F. nucleatum is always evident, selection of biospecimen could affect the role of F. nucleatum as a biomarker in CRC to predict and monitor tumor progression and prognosis as well as evaluate treatment effect.
Footnotes
Ethics Statement
Not applicable.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author Contributions
Conceptualization: YK. Methodology: YK, GHK. Resources: NYC. Writing—original draft: YK. Writing—review & editing: GHK. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This work was financially supported by a grant from the National Research Foundation (NRF) funded by the Korean Ministry of Science and ICT (2019R1F1A1061227) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1277).
Supplementary Information
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2022.03.13.
References
- 1.Wu Y, Wu J, Chen T, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis in mice via a Toll-like receptor 4/p21-activated kinase 1 cascade. Dig Dis Sci. 2018;63:1210–8. doi: 10.1007/s10620-018-4999-2. [DOI] [PubMed] [Google Scholar]
- 2.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–15. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015;137:1258–68. doi: 10.1002/ijc.29488. [DOI] [PubMed] [Google Scholar]
- 7.McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abed J, Emgard JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215–25. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-like receptor 4 signaling to nuclear factorkappaB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152:851–66. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–63. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandip KC, Steer CJ. Novel mechanisms of chemoresistance by Fusobacterium nucleatum involve not so novel pathways of microRNAs and autophagy. Transl Cancer Res. 2018;7(Suppl 1):S10–5. [Google Scholar]
- 13.Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–8. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Z, Cao S, Liu S, et al. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget. 2016;7:46158–72. doi: 10.18632/oncotarget.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2018;53:517–24. doi: 10.1007/s00535-017-1382-6. [DOI] [PubMed] [Google Scholar]
- 16.Yan X, Liu L, Li H, Qin H, Sun Z. Clinical significance of Fusobacterium nucleatum, epithelial-mesenchymal transition, and cancer stem cell markers in stage III/IV colorectal cancer patients. Onco Targets Ther. 2017;10:5031–46. doi: 10.2147/OTT.S145949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh HJ, Kim JH, Bae JM, Kim HJ, Cho NY, Kang GH. Prognostic impact of Fusobacterium nucleatum depends on combined tumor location and microsatellite instability status in stage II/III colorectal cancers treated with adjuvant chemotherapy. J Pathol Transl Med. 2019;53:40–9. doi: 10.4132/jptm.2018.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto Y, Mima K, Daitoku N, et al. Fusobacterium nucleatum in colorectal cancer liver metastasis and patient prognosis. Cancer Sci. 2018;109:1360. doi: 10.1111/cas.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernyavskiy P, Kennerley VM, Jemal A, Little MP, Rosenberg PS. Heterogeneity of colon and rectum cancer incidence across 612 SEER counties, 2000-2014. Int J Cancer. 2019;144:1786–95. doi: 10.1002/ijc.31776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Carvalho AC, de Mattos Pereira L, Datorre JG, et al. Microbiota profile and impact of Fusobacterium nucleatum in colorectal cancer patients of Barretos Cancer Hospital. Front Oncol. 2019;9:813. doi: 10.3389/fonc.2019.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into metaanalysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, An QM, Tian XY, et al. Fusobacterium nucleatum infection is correlated with tumor metastasis and postoperative survival of colorectal cancer patients in China. Transl Cancer Res. 2016;5:579–88. [Google Scholar]
- 23.Kunzmann AT, Proenca MA, Jordao HW, et al. Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients. Eur J Clin Microbiol Infect Dis. 2019;38:1891–9. doi: 10.1007/s10096-019-03649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Lu Y, Ke Y, Li Y. Prognostic impact of the Fusobacterium nucleatum status in colorectal cancers. Medicine (Baltimore) 2019;98:e17221. doi: 10.1097/MD.0000000000017221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–61. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Chen Y, Zhang J, et al. Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics. 2020;10:323–39. doi: 10.7150/thno.38870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Liu Y, Li J, et al. Fusobacterium nucleatum acts as a procarcinogenic bacterium in colorectal cancer: from association to causality. Front Cell Dev Biol. 2021;9:710165. doi: 10.3389/fcell.2021.710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Yang Y, Weng W, et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. 2019;38:14. doi: 10.1186/s13046-018-0985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae JM, Kim JH, Kang GH. Molecular subtypes of colorectal cancer and their clinicopathologic features, with an emphasis on the serrated neoplasia pathway. Arch Pathol Lab Med. 2016;140:406–12. doi: 10.5858/arpa.2015-0310-RA. [DOI] [PubMed] [Google Scholar]
- 30.Wen X, Jeong S, Kim Y, et al. Improved results of LINE-1 methylation analysis in formalin-fixed, paraffin-embedded tissues with the application of a heating step during the DNA extraction process. Clin Epigenetics. 2017;9:1. doi: 10.1186/s13148-016-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freifelder D, Davison PF. Physicochemical studies on the reaction between formaldehyde and DNA. Biophys J. 1963;3:49–63. doi: 10.1016/s0006-3495(63)86803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imrit K, Goldfischer M, Wang J, et al. Identification of bacteria in formalin-fixed, paraffin-embedded heart valve tissue via 16S rRNA gene nucleotide sequencing. J Clin Microbiol. 2006;44:2609–11. doi: 10.1128/JCM.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gethings-Behncke C, Coleman HG, Jordao HWT, et al. Fusobacterium nucleatum in the colorectum and its association with cancer risk and survival: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2020;29:539–48. doi: 10.1158/1055-9965.EPI-18-1295. [DOI] [PubMed] [Google Scholar]
- 34.Huangfu SC, Zhang WB, Zhang HR, et al. Clinicopathological and prognostic significance of Fusobacterium nucleatum infection in colorectal cancer: a meta-analysis. J Cancer. 2021;12:1583–91. doi: 10.7150/jca.50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.