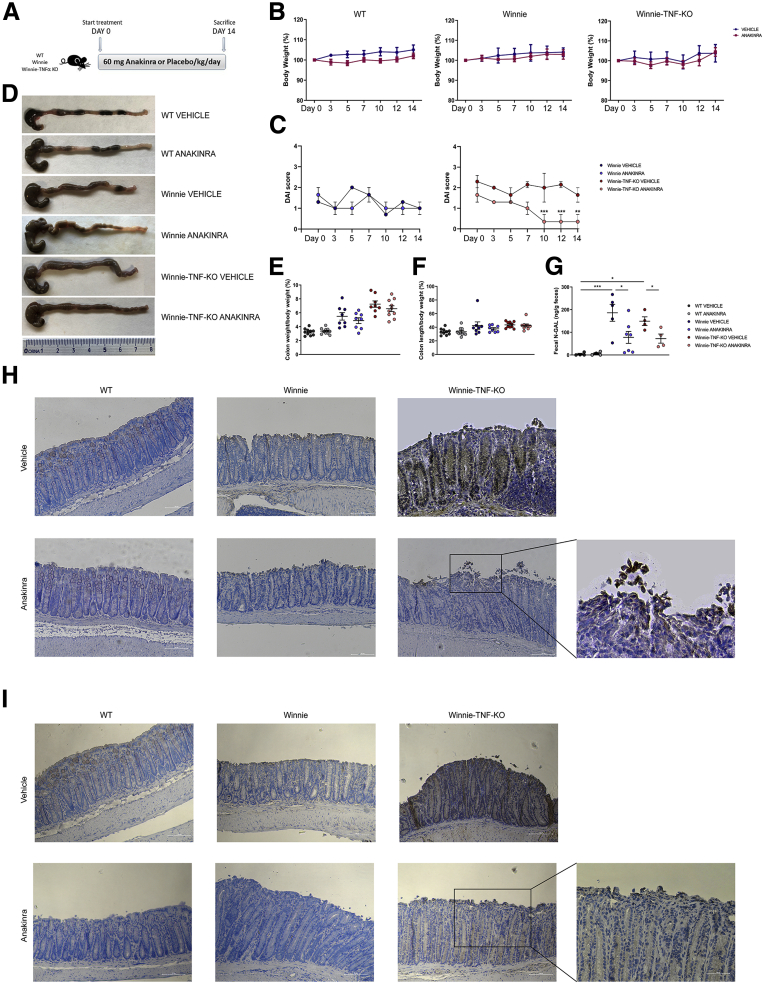
Figure 4.
Macroscopic features and measurements of colonic parameters at the end of treatment with anakinra. (A) Schematic representation of the experimental design. WT, Winnie and Winnie-TNF-KO mice were treated with IP injection of anakinra or vehicle for 14 days (N = 9 for each group). (B) Effect of anakinra or vehicle on mice body weight from day 0 until the end of treatment. (C) DAI score recorded for Winnie and Winnie-TNF-KO mice treated with anakinra or vehicle. (D) Representative images of whole colons for each experimental group. (E and F) Measurement of colon weight/body weight and colon length/body weight indices (%), respectively. (G) Detection of N-GAL level in feces of WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle. (H and I) IHC analysis for IL1β and caspase 8, respectively, in formalin-fixed, paraffin-embedded tissues obtained from WT, Winnie, and Winnie-TNF-KO mice treated with anakinra or vehicle. (10× and magnified 20× on the right). ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.