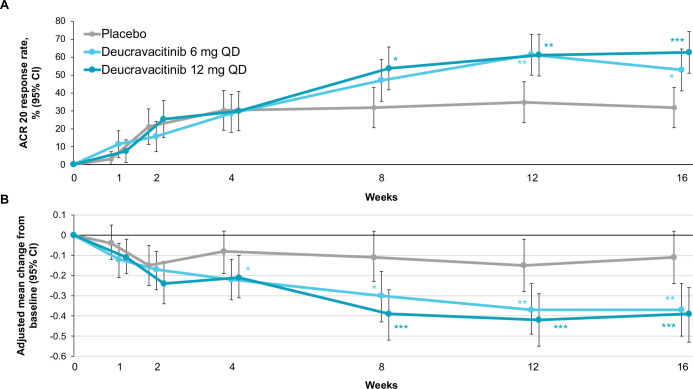
Figure 1.
ACR-20 response and change in HAQ-DI score over time. Supporting values are shown in online supplemental table S4. (A) Time course of ACR-20 response through week 16. Response rates are reported in the intention-to-treat population (ie, all randomised patients) with non-responder imputation; patients who discontinued the trial early, started a prohibited treatment, were lost to follow-up or had no ACR assessments had outcomes imputed as non-responses. (B) Adjusted mean change from baseline in HAQ-DI score through week 16. Placebo, n=66; deucravacitinib 6 mg once a day, n=70; deucravacitinib 12 mg once a day, n=67. P values indicate a difference from placebo: *p<0.05, **p≤0.01, ***p≤0.001, adjusted for multiplicity at week 16 only. ACR, American College of Rheumatology; HAQ-DI, Health Assessment Questionnaire-Disability Index; QD, once a day.