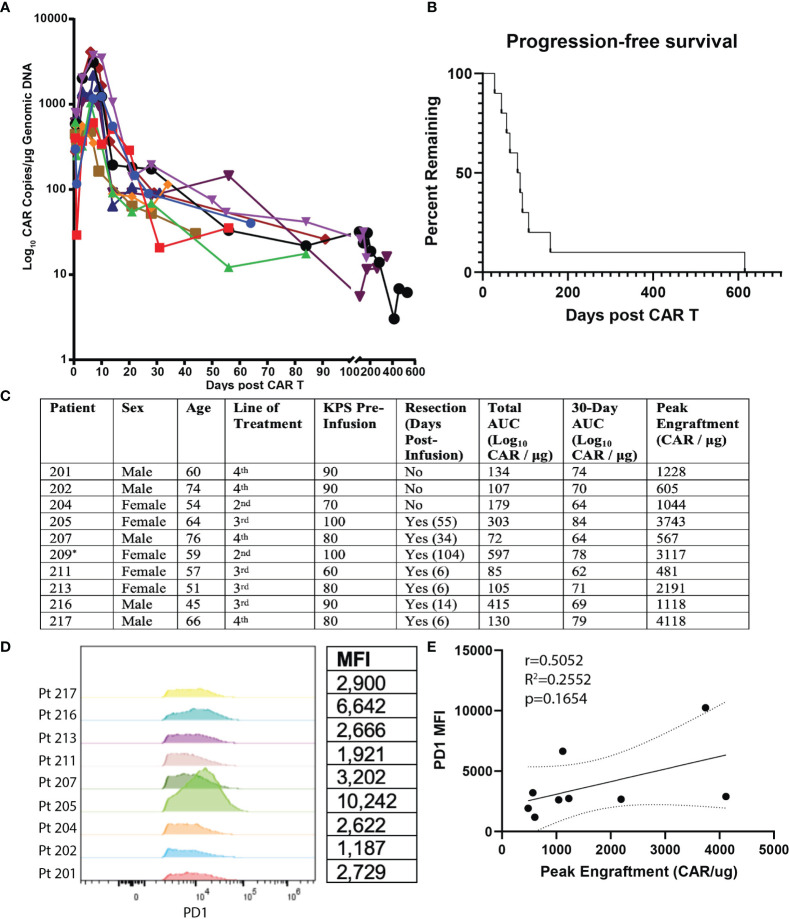
Figure 1.
Summary of Clinical Outcomes for EGFRvIII-Directed CAR T Clinical Trial and Flow Cytometric Analysis of Patient Plasma Products. Summary of clinical outcomes for clinical trial on EGFRvIII-directed CAR T for recurrent glioblastoma (NCT02209376). Patient 209 was ultimately not included for analysis due to having an outlier PFS value. (A) Plot of days following CAR T infusion and engraftment in peripheral blood. Following earlier studies, peripheral engraftment was quantified as log10copies/μg of genomic DNA. (B) PFS plotted as Kaplan-Meier estimator for all subjects. (C) Summary values of patient characteristics, performance of resection, total AUC, 30-day AUC, and peak engraftment for all subjects. * Patient 209 was excluded due to having an outlier progression-free survival (p<0.001 on Grubbs’ test). (D) Comparison of PD1 expression for CD3+CD4+CAR+ cells for transfusion products from all nine recurrent GBM patients receiving EGFRvIII-directed CAR T. Mean fluorescence intensity (MFI) is quantified on the table to the right. (E) Correlation of PD1 MFI and peak CAR T engraftment levels.