Figure 1.
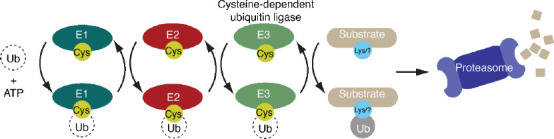
Cysteine-dependent ubiquitin ligases are key components of the ubiquitin-proteasome system.
Ubiquitin ligases operate within an enzymatic cascade consisting of E1 activating enzymes (E1s), E2 conjugating enzymes (E2s) and E3 ubiquitin ligases. Dependent on adenosine 5’-triphosphate (ATP), the E1 forms a reactive cysteine-linked intermediate with the C-terminal tail of ubiquitin. The Ub molecule is next passed onto a cysteine in an E2. Of the hundreds of ubiquitin ligase that exist, approximately 45 contain a single catalytic cysteine (transthiolation E3s) which accepts the Ub molecule from E2 via a transthiolation reaction. Protein substrates are then selected by the ubiquitin ligase and modified with Ub. This typically targets the substrate for degradation by the proteasome.
