Figure 2.
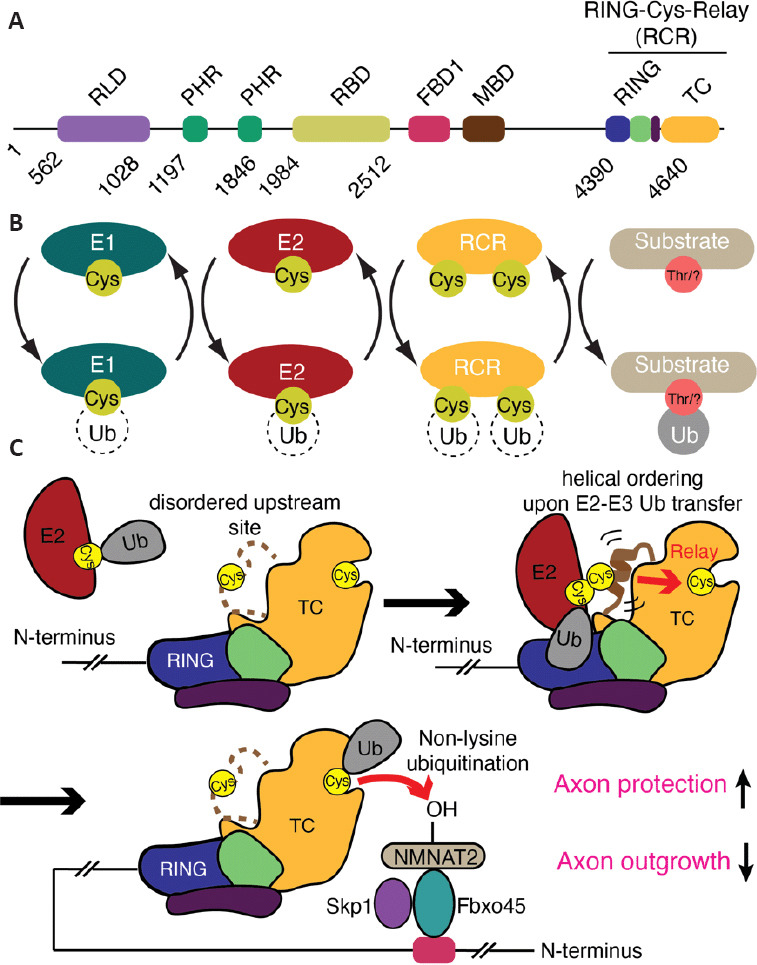
The mechanism of MYCBP2 RING-Cys-Relay activity and its role in axon protection and neural development.
(A) Domain architecture of MYCBP2 (RCC1-like GEF domain (RLD), two PHR-family-specific (PHR) domains, a RAE1 binding domain (RBD), an F-box binding domain 1 (FBD1), a Myc binding domain (MBD), C-terminal RING domain (RING) and Tandem Cysteine Domain (TC) that contains two active site cysteine residues involved in substrate ubiquitination. (B) Reaction scheme depicting how the RCR module within MYCBP2 operates within the multienzyme ubiquitin cascade. (C) The unusual ubiquitin E3 ligase MYCBP2 binds a ubiquitin-charged E2 enzyme via its RING domain. The ubiquitin molecule is transferred to an upstream catalytic cysteine within the TC domain which transiently induces local ordering of the upstream site. This might serve as an “entropic spring” allowing the ubiquitin molecule to be relayed to a downstream catalytic cysteine. Although not experimentally confirmed, the working model is that Fbxo45 and Skp1 bind to the distal N-terminal FBD1 region. They are responsible for binding substrates, including NMNAT2, and position them proximal to the downstream cysteine within the RCR module. The downstream site efficiently ubiquitinates hydroxy amino acids rather than conventional lysine residues. This unconventional mechanism of ubiquitination involving the two catalytic cysteines, termed RING-Cys-Relay (RCR), is central to neural development and its loss confers neurite protection in primary superior cervical ganglion cultures.
