Figure 2. CONSORT Diagram of the GeparX Study.
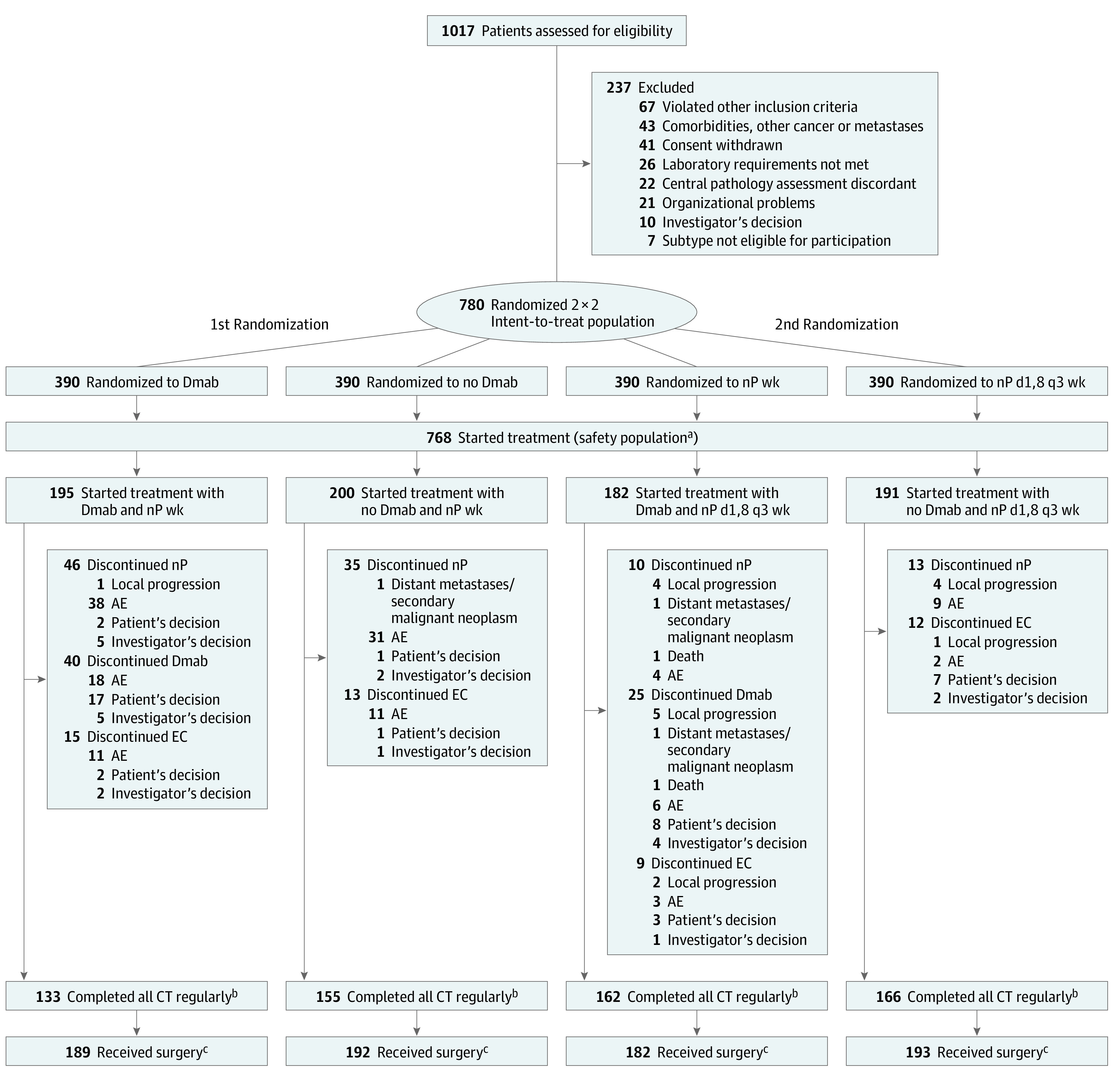
AE indicates adverse event; CT, chemotherapy; Dmab, denosumab; EC, epirubicin and cyclophosphamide; nP, nab-paclitaxel; wk, weekly.
aThree patients did not start Dmab and were analyzed for safety and adherence in no Dmab arm; in 7 patients of nP d1,8 q3 wk arm, there was no pause at day 15 in at least 2 cycles, and they were analyzed for safety and adherence in the nP weekly arm.
bIncludes patients who completed both nP and EC.
cOf patients “as randomized.”
