Fig. 6. FDA-approved sialidase inhibitor oseltamivir blocks AMR-mediated platelet clearance and protects against SA bacteremia.
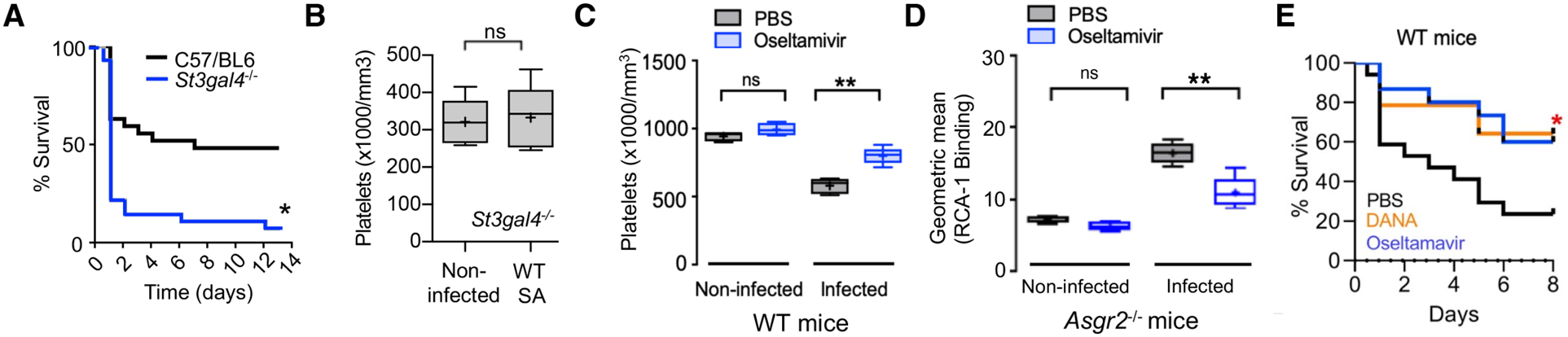
(A) St3gal4−/− mice that have decreased platelet sialylation and thrombocytopenia show accelerated mortality upon SA bloodstream infection (n = 10 per group). (B) circulating platelet count 4 h after IV SA challenge in WT vs. St3gal4−/− mice (n = 10 per group). (C) Platelets isolated from Asgr2−/− mice treated with or without oseltamavir and infected with MRSA were assessed for RCA-1 lectin binding. (D) C57/Bl6 mice were treated with oseltamavir (n = 6) or PBS control (n = 5) and infected with WT SA by intraperitoneal injection. Blood was harvested 24 h after infection and platelet counts collected. (E) 8-day mortality study conducted on C57/Bl6 mice treated with DANA (n = 16), oseltamavir (n = 16), or PBS control (n = 16). Statistical significance was determined by unpaired two-tailed Student’s t-test (B), two-way ANOVA with Bonferroni’s multiple comparisons posttest (C,D), or Log-rank (Mantel-Cox) Test (A,E). For floating bar graphs, + denotes the mean, whiskers represent min. to max, and floating box represents 25th to 75th percentile. Unless otherwise stated, *P < 0.05, **P < 0.005. PBS, phosphate buffered saline; ns, not significant.
