Abstract
Background
The applicability of pulse pressure variation (ΔPP) to predict fluid responsiveness using lung-protective ventilation strategies is uncertain in clinical practice. We designed this study to evaluate the accuracy of this parameter in predicting the fluid responsiveness of septic patients ventilated with low tidal volumes (TV) (6 ml kg−1).
Methods
Forty patients after the resuscitation phase of severe sepsis and septic shock who were mechanically ventilated with 6 ml kg−1 were included. The ΔPP was obtained automatically at baseline and after a standardized fluid challenge (7 ml kg−1). Patients whose cardiac output increased by more than 15% were considered fluid responders. The predictive values of ΔPP and static variables [right atrial pressure (RAP) and pulmonary artery occlusion pressure (PAOP)] were evaluated through a receiver operating characteristic (ROC) curve analysis.
Results
Thirty-four patients had characteristics consistent with acute lung injury or acute respiratory distress syndrome and were ventilated with high levels of PEEP [median (inter-quartile range) 10.0 (10.0–13.5)]. Nineteen patients were considered fluid responders. The RAP and PAOP significantly increased, and ΔPP significantly decreased after volume expansion. The ΔPP performance [ROC curve area: 0.91 (0.82–1.0)] was better than that of the RAP [ROC curve area: 0.73 (0.59–0.90)] and pulmonary artery occlusion pressure [ROC curve area: 0.58 (0.40–0.76)]. The ROC curve analysis revealed that the best cut-off for ΔPP was 6.5%, with a sensitivity of 0.89, specificity of 0.90, positive predictive value of 0.89, and negative predictive value of 0.90.
Conclusions
Automatized ΔPP accurately predicted fluid responsiveness in septic patients ventilated with low TV.
Keywords: fluid therapy; haemodynamics; respiratory distress syndrome, adult; sepsis; tidal volume
Editor's key points.
-
•
Changes in cardiac output were measured after fluid challenges in intensive care unit septic patients.
-
•
Pulse pressure variability before and after fluid challenges were measured during 6 and 8 ml kg−1 tidal volume ventilation.
-
•
Importantly, pulse pressure variation was a better predictor of fluid responsiveness than the static indicators.
After early sepsis resuscitation, excessive fluid administration may aggravate pulmonary oedema and prolong mechanical ventilation.1 An accurate prediction of fluid responsiveness may prevent unnecessary fluid loading and detect patients who benefit from volume expansion.2
Previous studies demonstrated that pulse pressure variation (ΔPP) is an accurate predictor of fluid responsiveness during mechanical ventilation.3 4 Almost all patients in these trials were ventilated with tidal volumes (TV) of 8–10 ml kg−1.5 However, low TV ventilation is commonly used in patients with sepsis because sepsis predisposes patients to acute lung injury/acute respiratory distress syndrome (ALI/ARDS).6 Ventilation with a low TV is usually considered a limitation for the assessment of functional haemodynamics.7 The rationale is that a low TV might be insufficient to produce a significant change in the intrathoracic pressure; therefore, ΔPP could indicate a non-responsive status even in ‘responders'.8
Previous clinical and experimental studies have conflicting results regarding the accuracy of ΔPP measured with a TV below 8 ml kg−1.9, 10, 11, 12 Furthermore, most studies calculated the ΔPP manually using a computer recording or paper print-out of the pressure curve, but this form of measurement has been criticized.13 Thus, the role of automatized ΔPP in this setting is of particular interest.
We designed a prospective study to evaluate the predictive value of automatized ΔPP for fluid responsiveness in patients with sepsis and low TV ventilation.
Methods
The institutional Research and Ethics Committee approved the study. The patients' closest relatives signed the informed consent form to allow the data collection.
This study was performed in a 14-bed mixed intensive care unit at a Brazilian teaching hospital. The inclusion criteria were as follows: age >18 yr, a diagnosis of severe sepsis or septic shock according to the criteria of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference,14 sedation and mechanical ventilation with a low TV (5.5–6.5 ml kg−1 of predicted body weight), instrumentation with indwelling radial or femoral artery and pulmonary artery catheters, a required fluid challenge (as determined by the attending physician), and a signed informed consent. We chose septic patients because they are usually monitored with pulmonary arterial catheter and respiratory dysfunction is often present, leading to ventilation with low TV.
All patients were included after the first 6 h of resuscitation as in this late phase, fluid responsiveness assessment is more relevant.15 The absence of spontaneous respiratory movements was identified upon clinical examination, and the respiratory curves were examined using the ventilator and capnographic signal on the bedside monitor. Patients received neuromuscular block if needed.
The exclusion criteria were as follows: cardiac arrhythmias and previously known significant valvular disease or intracardiac shunt, acute bleeding (suspected or confirmed), air leakage through chest drains, an urgently required fluid challenge, abdominal compartment syndrome, and pregnancy.
Baseline and sepsis-related characteristics, and also the Acute Physiological and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA) severity scores, were collected at the patient's inclusion.
Study protocol
The selected patients were mechanically ventilated (Vela, Viasys, Palm Springs, CA, USA) using the volume-controlled mode; the patients' TV was adjusted to 6 ml kg−1 (based on the patient's predicted body weight), with no changes in the other ventilatory parameters. The predicted body weight of male patients was calculated as equal to 50+0.91 (centimetres of height−152.4); that of female patients was calculated as equal to 45.5+0.91 (centimetres of height−152.4).16 The static compliance of the respiratory system was calculated as follows: TV/(plateau pressure−PEEP). The plateau pressure was measured after an inspiratory pause of 2 s.
Throughout the study period, the doses of the sedative, inotropic, and vasopressor medications remained constant. Each patient was observed for 20 min before the fluid challenge to assure that there were no significant variations in haemodynamic parameters. If the heart rate (HR), ΔPP, arterial pressure, right atrial pressure (RAP), pulmonary arterial occlusion pressure (PAOP), or cardiac output (CO) varied by more than 20% during this period of observation, the experiment was interrupted. At the end of 20 min (baseline), we obtained a complete set of haemodynamic and respiratory measurements, including arterial and mixed-venous blood gases, haemoglobin, and arterial lactate levels. At this time, the ΔPP was measured in patients ventilated with a TV of 6 ml kg−1 and was recorded as ΔPP6.
To assess the correlation and the agreement between the ΔPP measured during low TV ventilation (6 ml kg−1) and during ‘standard' TV ventilation (8 ml kg−1), we increased the TV to 8 ml kg−1 of predicted body weight. After 5 min, the haemodynamic and respiratory measurements were repeated. The ΔPP measured at this time was recorded as ΔPP8. No fluids were given at this step.
After this manoeuvre, the patients were again ventilated with a TV of 6 ml kg−1 and given a standardized fluid challenge with 7 ml kg−1 (actual body weight) of hydroxylethyl starch 130/0.4 (up to 500 ml), which was infused over 30 min. At the end of the fluid challenge, another set of haemodynamic and respiratory measurements was obtained.
Considering the CO obtained with a TV of 6 ml kg−1, we classified the patients into two groups according to their per cent increase in CO in response to the fluid challenge. ‘Responders' had a CO increase of at least 15%, whereas ‘non-responders' had a CO increase of <15%.3 17 The CO was determined by a semicontinuous thermodilution technique that considered the average value of four consecutive measurements from the STAT mode screen of the Vigilance® monitor (Edwards, Irvine, CA, USA). The ΔPP was measured with a multiparameter bedside monitor (DX 2020, Dixtal, São Paulo, Brazil) using an automatic calculation and real-time monitoring of ΔPP. The monitor uses specific software allowing the recognition of respiratory cycles (capnographic signal) and the automatic calculation of ΔPP over each respiratory cycle. The mean value of ΔPP is calculated over three consecutive periods of 10 respiratory cycles (from cycles 1 to 10, 2 to 11, and 3 to 12); the median value of this triple determination is displayed on the bedside monitor. This automatic real-time monitoring of ΔPP was validated previously in patients using TV of 8 ml kg−1 and a PEEP of 5 cm H2O.18 All pressures were determined at the end-expiration with the zero reference level settled at 4th–5th intercostal space along the mid-axillary line. The head of the bed was elevated at ∼30°.
Statistical analysis
Categorical variables were compared using the Pearson χ 2 test. The distribution of continuous variables was assessed by a Shapiro–Wilk test, and variance homogeneity was assessed with a Bartlett test. The data that were normally distributed and had a homogenous variance were expressed by means [standard deviations (sd)]. Non-parametric variables were described as medians and inter-quartile ranges (IQR). The effects of intravascular volume expansion on haemodynamic variables were assessed using a Wilcoxon's rank-sum test or a paired t-test, as appropriate. The haemodynamic variables before the fluid challenge in responders and non-responders were compared using the Mann–Whitney U-test or a t-test, as appropriate.
Changes in CO after the fluid challenge were expressed as percentages. The correlation between changes in CO and ΔPP6 was assessed using the best curve estimation model. Receiver operator characteristic (ROC) curves were constructed to evaluate the capacity of ΔPP6, PAOP, and RAP to predict fluid responsiveness. The best cut-off values were calculated for all variables. A predefined subgroup analysis was performed according to driving pressure and PEEP levels. In this analysis, the median was the cut-off value, and ROC curves were constructed for each subgroup (patients with high- and low-driving pressure and PEEP). We compared the area under the ROC curves in each of these subgroups using an unpaired t-test.
To analyse whether changing the TV to 8 ml kg−1 could accurately predict that a given patient would be fluid responsive while ventilated at 6 ml kg−1, an ROC curve was constructed for ΔPP8 that considered the CO response obtained after the fluid challenge, which was conducted while the patient was ventilated at 6 ml kg−1. The best cut-off value was estimated. The percentage of correct classification of both ΔPP6 and ΔPP8 in a given patient was calculated, and the agreement between ΔPP6 and ΔPP8 was compared using a marginal homogeneity test.
SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA) were used to conduct the statistical analysis. The results with P-values of <0.05 were considered significant.
Results
Forty patients were included from August 2007 to February 2009. Thirty-eight patients (95%) had septic shock. The lung was the most common site of infection. The main indications for fluid challenge were to reduce vasopressors (60%), hypotension (20%), and hyperlactataemia (12.5%). Six patients were not included because their arterial pressure varied more than 20% during the observation period. No patient had changes in CO >10% during this 20 min period. The patient characteristics are available in Table 1 .
Table 1.
Patient characteristics. APACHE II, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment score; ALI/ARDS, acute lung injury/acute respiratory distress syndrome; ICU, intensive care unit. Data are presented as the means (sd) or medians (IQR: 25th–75th percentile)
| Variable | Global |
|---|---|
| Number (n) | 40 |
| Age (yr) | 60 (49–76) |
| Gender [male (%)] | 28 (70%) |
| Predicted body weight (kg) | 60.9 (9.5) |
| APACHE II score | 21 (15–25.5) |
| SOFA score | 11 (8.5–12) |
| Septic shock [n (%)] | 38 (95) |
| Source of infection | |
| Lungs [n (%)] | 14 (35) |
| Intra-abdominal [n (%)] | 12 (30) |
| Others [n (%)] | 7 (17.5) |
| Not identified [n (%)] | 7 (17.5) |
| Category, medical (%) | 12 (30) |
| Survivors (ICU) [n (%)] | 15 (37.5) |
Thirty-four patients had characteristics consistent with ALI/ARDS.19 The median (IQR) compliance and PaO2/F I O2 ratio of all patients were 31 ml cm H2O (23.6–37.9) and 216.6 mm Hg (156.5–306.9), respectively. The mean (sd) respiratory rates (RRs) were 17.5 (1.9) cycles min−1, and the HR/RR ratio was 6.0 (5.3–7.1). The median PEEP was 10 (10–13.5) cm H2O. All patients were deeply sedated (Ramsay sedation scale: 6) and eight patients were paralysed.
Twenty-one patients did not have a CO increase above 15% after the fluid challenge; among them, six presented with a decline in CO, with a maximum reduction of 7.02% (CO from 11.4 to 10.6). Nineteen patients showed an increased CO of 15% or more after the fluid challenge (47.5%); three of these patients had a high increase (>50%). Age, gender, predicted body weight, the APACHE II score, and SOFA score were similar between responders and non-responders (data not shown).
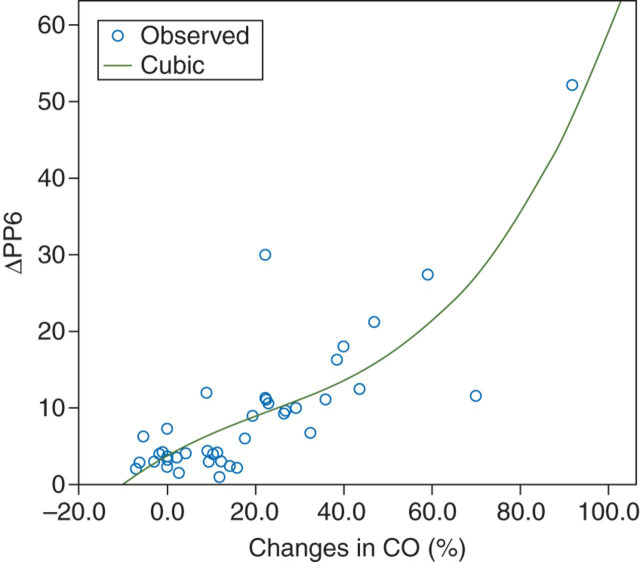
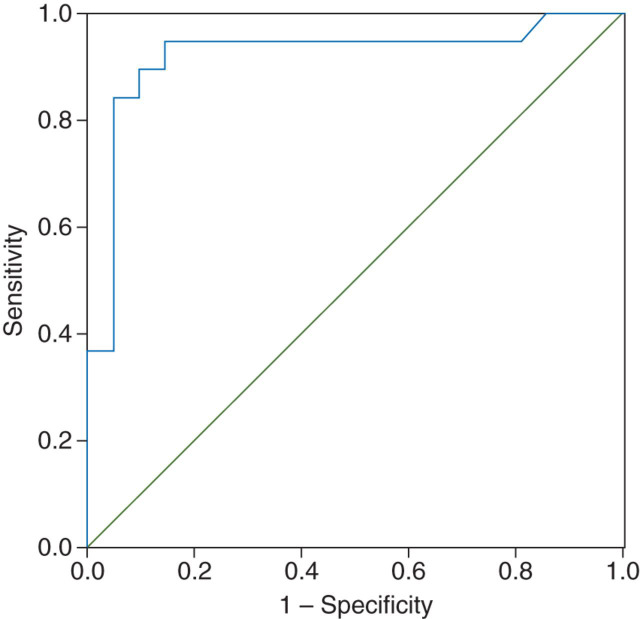
The respiratory and haemodynamic variables are presented in Table 2 . The best model approach noted a significant cubic relationship between ΔPP6 and changes in CO after the fluid challenge (r=0.71) (Fig. 1 ). Overall, the ΔPP6 before the fluid loading was 6.1% (3.0–11.2%). As expected, static variables were poor predictors of fluid responsiveness. The areas under the ROC curve were 0.58 (0.40–0.76) and 0.73 (0.59–0.90), and the cut-off values were 11.8 and 12.5 mm Hg for PAOP and RAP, respectively. However, the area under the ROC curve for ΔPP6 was 0.91 (0.82–1.0), demonstrating that this was a good parameter to predict fluid responsiveness (Fig. 2 ). The best cut-off for ΔPP6 was 6.5% with a sensitivity of 0.89, specificity of 0.90, positive predictive value of 0.89, negative predictive value of 0.90, and percentage of correct classification of 90%.
Table 2.
Respiratory and haemodynamic variables before and after fluid. ΔPP6, pulse pressure variation (6 ml kg−1); ΔPP8, pulse pressure variation (8 ml kg−1); HR, heart rate; MAP, mean arterial pressure; PAP, mean pulmonary arterial pressure; RAP, right atrial pressure; PAOP, pulmonary artery occlusion pressure; CO, cardiac output; SvO2, mixed-venous oxygen saturation; PEEP, positive end-expiratory pressure; PP, plateau pressure; Cstat, static respiratory compliance; DP, driving pressure; RR, respiratory rate. Data are presented as the means (sd) or medians (IQR: 25th–75th percentile). *P<0.05 vs baseline. †P<0.05 vs ‘responders'
| Variable | Responders (n=19) |
Non-responders (n=21) |
||
|---|---|---|---|---|
| Before fluid | After fluid | Before fluid | After fluid | |
| ΔPP6 (%) | 11.1 (9.3–18.0) | 4.5 (2.5–7.1)* | 3.5 (2.6–4.2)† | 2.6 (1.2–3.6)* |
| ΔPP8 (%) | 17.5 (12.7–24.9) | — | 5.7 (4.4–8.5)† | — |
| HR (beats min−1) | 115 (18) | 113 (19) | 100 (25)† | 100 (23) |
| MAP (mm Hg) | 69 (8) | 80 (11)* | 70 (6) | 78 (10)* |
| PAP (mm Hg) | 29 (5) | 33 (6)* | 29 (5) | 34 (4)* |
| RAP (mm Hg) | 11 (3) | 14 (3)* | 13 (3)† | 18 (2)* |
| PAOP (mm Hg) | 11 (4) | 14 (3)* | 12 (3) | 17 (3)* |
| CO (litre min−1) | 6.1 (2.2) | 8.2 (2.6)* | 7.1 (2.6) | 7.3 (2.6) |
| SvO2 (%) | 72.0 (70.0–75.5) | 80.0 (68.0–83.3) * | 71.4 (66.5–73.7) | 67.9 (65.2–75.7) |
| Lactate (mg dl−1) | 16 (9–34) | 15 (8–33)* | 18 (11–30) | 16 (10–27) |
| PEEP (cm H2O) | 10.0 (10.0–16.0) | — | 12.0 (9.5–13.0) | — |
| PP (cm H2O) | 22 (4) | 23 (5) | 25 (5) | 25 (4)* |
| Cstat (ml cm H2O−1) | 33.9 (7.4) | 32.9 (8.0) | 29.3 (10.7) | 27.8 (9.3)* |
| DP (cm H2O) | 11.0 (9.0–13.0) | 12.0 (9.0–14.0) | 13.0(10.5–17.5)† | 13.0 (11.5–18.0)* |
| RR (cycles min−1) | 18 (16–18) | — | 18.0 (17.0–20.0) | — |
| HR/RR | 6.9 (1.2) | 6.7 (1.3) | 5.5 (1.2)† | 5.5 (1.1) |
| PaO2/FIO2 (kPa) | 31.1 (10.3) | 37.9 (8.7) | 28.5 (10.6) | 29.4 (11.9) |
Fig 1.
Curvilinear relationship between ΔPP6 at baseline and changes in CO after the fluid challenge. ΔPP6, pulse pressure variation (6 ml kg−1); CO, cardiac output. R2: 0.71, P<0.001.
Fig 2.
ROC curve for ΔPP6. The ROC curve area was 0.91 (0.05) (P<0.001).
The driving pressure and PEEP levels did not influence the areas under the ROC curves [0.94 (0.86–1.0) and 0.91 (0.73–1.0), P=0.64, for lower and higher driving pressure subgroups and 0.99 (0.96–1.0) and 0.84 (0.62–1.0), P=0.17, for lower and higher PEEP levels subgroups].
The ΔPP increased in all patients after changing the TV to 8 ml kg−1 of the predicted body weight, except in one patient who was non-responsive to the fluid challenge (ΔPP6: 4.1%, ΔPP8: 3.8%, CO variation: 4.2%). Overall, the ΔPP8 was 9.7% (5.6–17.2%). A significant linear correlation between ΔPP6 and ΔPP8 was also observed (r=0.92). The median difference between ΔPP6 and ΔPP8 was 3.9% (2.0–5.9%). The best cut-off for ΔPP8 was 12.3%, with a sensitivity of 0.80, specificity of 0.95, positive predictive value of 0.93, and negative predictive value of 0.95 and percentage of correct classification of 87.5% to predict the fluid responsiveness under a TV of 6 ml kg−1. There were no statistically significant differences between the proportions of correct classification in the ΔPP8 and ΔPP6 groups (P=0.65).
Discussion
The main finding of this study was that automatized ΔPP is a reliable marker of fluid responsiveness in septic patients with ALI/ARDS ventilated with a low TV. A cut-off value of 6.5% may be applied to distinguish ‘responders' from ‘non-responders' in this scenario.
Ventilation with a low TV is usually considered a limitation for reliable analysis of respiratory changes in arterial pressure.7 However, the influence of a low TV on functional haemodynamic parameters is still a matter of debate.13 Previous studies in patients ventilated with a low TV demonstrated that the baseline ΔPP was significantly correlated with cardiac index changes in response to fluid loading, whereas neither baseline values of RAP nor PAOP revealed a significant correlation.20 21 The opposite has also been described, as studies examining the predictive value of ΔPP in patients suffering from various critical illnesses reported a low accuracy for ΔPP when the TV was <8 ml kg−1.9 22 These authors did not use a high and low TV for the same patient but rather analysed the patients at the TVs with which they were already being ventilated. Thus, patients in the lower TV subgroup (<8 ml kg−1) more often presented with ALI/ARDS. In these patients, other factors may also compromise the accuracy of the ΔPP rather than TV, such as an increased respiratory rate or the presence of pulmonary hypertension, right ventricular dysfunction, or both.23 24 The differing cut-off values for ΔPP among these studies (5–12% in ARDS patients) reinforce this hypothesis.9, 10, 11
In addition to the aforementioned factors, the reduction in functional residual capacity may contribute to the poor performance of ΔPP in patients with ALI/ARDS. The total lung volume of these patients is severely reduced when compared with normal subjects.25 Wiklund and colleagues suggested in an animal study that ΔPP was a reliable indicator of severe hypovolaemia in pigs with healthy lungs regardless of the TV. In contrast, in pigs with an ARDS-like syndrome ventilated with a low TV and low PEEP, the ΔPP was not a good indicator of hypovolaemia.26 However, another animal study demonstrated that ΔPP was a good predictor of hypovolaemia in pigs with ALI ventilated with a low TV and elevated PEEP levels.12 The poorest performance of the ΔPP in previous experimental and clinical studies may be partly attributed to the reduced PEEP levels, as an increase in the functional residual capacity is a desirable effect of PEEP. In a study involving patients with ARDS ventilated with a low TV [6.4 (0.7) ml kg−1] and high PEEP [13.9 (1.4) cm H2O], the performance of ΔPP was better.11 As suggested by the authors, high PEEP levels, by exaggerating the cyclic changes in pleural pressures, may reduce the disadvantageous effects of low TV in predicting fluid responsiveness. Thus, a possible explanation for the good performance of ΔPP6 would be the higher PEEP levels used in our patients together with a lower respiratory frequency (Table 2). Although we were unable to corroborate this hypothesis in our subgroup analysis of PEEP levels, this could be only a consequence of the reduced number of individuals in the PEEP subgroups.
Another possible explanation for our results would be that, unlike previous studies, we used a multiparameter bedside monitor to automatically calculate the ΔPP. There are concerns regarding the manual ΔPP estimation used in the previous studies. First, small errors in the pressure measurements are more frequent when performed manually. Pulse pressure variation has a low amplitude; therefore, artifacts and noise errors are more common.13 Secondly, only three consecutive measurements are commonly averaged to determine the ΔPP. Kim and Pinsky27 demonstrated that increasing the sampling duration to include more positive-pressure breaths increases the magnitude of the calculated ΔPP. An automatized ΔPP has also been criticized, in part due to its lack of standardization;28 however, automatized ΔPP may represent an advance in clinical practice.13 28 This same automatized ΔPP algorithm was previously tested in two studies in patients during heart surgery and non-cardiac major surgery.18 29 The area under the ROC curve in one of these studies was 0.98 and the automated method had a sensitivity of 97% and a specificity of 95% to predict fluid responsiveness.18 Other similar automated algorithms were validated in other studies.30 31
We also demonstrated that the ΔPP6 was highly correlated with the ΔPP8. Interestingly, the cut-off of the ΔPP8 (12.3%) to discriminate the ‘responders' from the ‘non-responders', which was defined by the CO improvement while ventilated at a TV of 6 ml kg−1, was similar to the cut-off value found in previous studies that conducted a fluid challenge under a TV of 8 ml kg−1.5 Although an increase in TV would probably change the haemodynamic status (producing a leftward shift on the Frank–Starling curve),32 we were able to demonstrate that, even under this potential interference, the ΔPP8 with a cut-off of 12.3% could predict a fluid response when the patient is returned to a TV of 6 ml kg−1. In other words, measuring the ΔPP6 or increasing the TV to 8 ml kg−1 are reasonable strategies for a fluid response with a TV of 6 ml kg−1 in patients with ALI/ARDS.
Our study has several strengths. In contrast to others,9 10 22 we evaluated patients using a fixed TV. For a given volume status, a different TV will lead to different ΔPP values, regardless of fluid responsiveness.27 33 Therefore, the determination of a clinical cut-off point through ROC curve analyses may be imprecise when different TVs are used. We standardized the amount of fluid infused because 500 ml of fluid represents a different load in a 50 kg patient than in a 100 kg patient. We also standardized the TV measurement based on the predicted body weight according to gender and height, whereas some studies did not specify how the TV was estimated.9 All of these factors might have contributed to the improved ΔPP6 accuracy in this study.
The main limitation of our study was the semicontinuous thermodilution technique for determining the CO. This method may underestimate the changes in CO if it is measured immediately after the fluid infusion.34 Although we waited 5 min after the fluid challenge to acquire the CO data, the results may be biased (i.e. borderline true ‘responders' could be falsely classified as ‘non-responders'). However, we evaluated these data for all patients and concluded that only two patients had borderline changes in CO, using a 15% change in CO as a cut-off. We analysed the results by considering these two patients to be ‘responders' and excluding them from the analysis. In both situations, the results were maintained (data not shown). A cut-off of 15% change in CO to differentiate ‘responders' from ‘non-responders' was used in our study, rather than any other change. This cut-off has been generally adopted in similar studies of functional haemodynamic monitoring to suggest clinical significance.5
Another potential limitation is that the automated ΔPP algorithm has never been studied at low TV. However, ventilation under low TV could lead to a decrease in pulse pressure. In this context, manual ΔPP calculation would be markedly affected by small errors in pressure measurements. This effect could be theoretically reduced during the automated measurement. Also, our study did not address how different ventilator variables influence ΔPP accuracy. Thus, this good performance of ΔPP6 needs to be confirmed in a larger sample including patients with ARDS ventilated with different PEEP levels and RRs. The essence of our findings is that there are factors other than TV that might interfere with the discriminative properties of ΔPP.
To conclude, our study evaluated septic patients with characteristics consistent with ALI/ARDS ventilated using protective strategies. Unlike previous studies, the accuracy of ΔPP was adequate in these patients when they were ventilated with a TV of 6 ml kg−1. We demonstrate that automatized ΔPP is a useful predictor of fluid responsiveness in patients with characteristics similar to ours. A cut-off value of 6.5% may be applied to discriminate between the ‘responders' and ‘non-responders' in this scenario.
Declaration of interest
None declared.
Funding
This work was fully supported by institutional funding.
Acknowledgements
We thank Isac Castro and Julia Fukushima for their support with the statistical analyses.
Handling editor: R. P. Mahajan
References
- 1.Michard F, Descorps-Declere A, Lopes M. Using pulse pressure variation in patients with acute respiratory distress syndrome. Crit Care Med. 2008;36:2946–2948. doi: 10.1097/CCM.0b013e318187b6fd. [DOI] [PubMed] [Google Scholar]
- 2.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 3.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 4.Preisman S, Kogan S, Berkenstadt H, Perel A. Predicting fluid responsiveness in patients undergoing cardiac surgery: functional haemodynamic parameters including the Respiratory Systolic Variation Test and static preload indicators. Br J Anaesth. 2005;95:746–755. doi: 10.1093/bja/aei262. [DOI] [PubMed] [Google Scholar]
- 5.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 6.Matthay M, Zimmerman G, Esmon C, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 7.Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–428. doi: 10.1097/00000542-200508000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Pinsky MR. Using ventilation-induced aortic pressure and flow variation to diagnose preload responsiveness. Intensive Care Med. 2004;30:1008–1010. doi: 10.1007/s00134-004-2208-6. [DOI] [PubMed] [Google Scholar]
- 9.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent J. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–523. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 10.Lakhal K, Ehrmann S, Benzekri-Lefèvre D, et al. Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care. 2011;15:R85. doi: 10.1186/cc10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Fu J, Hu H, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008;36:2810–2816. doi: 10.1097/CCM.0b013e318186b74e. [DOI] [PubMed] [Google Scholar]
- 12.da Silva Ramos FJ, de Oliveira EM, Park M, Schettino GP, Azevedo LC. Heart–lung interactions with different ventilatory settings during acute lung injury and hypovolaemia: an experimental study. Br J Anaesth. 2011;106:394–402. doi: 10.1093/bja/aeq404. [DOI] [PubMed] [Google Scholar]
- 13.Teboul J, Vieillard-Baron A. Clinical value of pulse pressure variations in ARDS. Still an unresolved issue? Intensive Care Med. 2005;31:499–500. doi: 10.1007/s00134-005-2587-3. [DOI] [PubMed] [Google Scholar]
- 14.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 15.Monnet X, Teboul JL. Volume responsiveness. Curr Opin Crit Care. 2007;13:549–553. doi: 10.1097/MCC.0b013e3282ec68b2. [DOI] [PubMed] [Google Scholar]
- 16.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 17.Stetz CW, Miller RG, Kelly GE. Reliability of the thermodilution method in the determination of cardiac output in clinical practice. Am Rev Respir Dis. 1982;126:1001–1004. doi: 10.1164/arrd.1982.126.6.1001. [DOI] [PubMed] [Google Scholar]
- 18.Auler JO, Galas F, Hajjar L, Santos L, Carvalho T, Michard F. Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg. 2008;106:1201–1206. doi: 10.1213/01.ane.0000287664.03547.c6. [DOI] [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 20.Wiesenack C, Fiegl C, Keyser A, Prasser C, Keyl C. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur J Anaesthesiol. 2005;22:658–665. doi: 10.1017/s0265021505001092. [DOI] [PubMed] [Google Scholar]
- 21.Marx G, Cope T, McCrossan L, et al. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol. 2004;21:132–138. doi: 10.1017/s0265021504002091. [DOI] [PubMed] [Google Scholar]
- 22.Vallée F, Richard JC, Mari A, et al. Pulse pressure variations adjusted by alveolar driving pressure to assess fluid responsiveness. Intensive Care Med. 2009;35:1004–1010. doi: 10.1007/s00134-009-1478-4. [DOI] [PubMed] [Google Scholar]
- 23.De Backer D, Taccone FS, Holsten R, Ibrahimi F, Vincent JL. Influence of respiratory rate on stroke volume variation in mechanically ventilated patients. Anesthesiology. 2009;110:1092–1097. doi: 10.1097/ALN.0b013e31819db2a1. [DOI] [PubMed] [Google Scholar]
- 24.Wyler von Ballmoos M, Takala J, Roeck M, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010;14:R111. doi: 10.1186/cc9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ. A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med. 1998;158:1644–1655. doi: 10.1164/ajrccm.158.5.9802003. [DOI] [PubMed] [Google Scholar]
- 26.Wiklund CU, Morel DR, Orbring-Wiklund H, et al. Influence of tidal volume on pulse pressure variations in hypovolemic ventilated pigs with acute respiratory distress-like syndrome. Anesthesiology. 2010;113:630–638. doi: 10.1097/ALN.0b013e3181e908f6. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Pinsky M. Effect of tidal volume, sampling duration, and cardiac contractility on pulse pressure and stroke volume variation during positive-pressure ventilation. Crit Care Med. 2008;36:2858–2862. doi: 10.1097/CCM.0b013e3181865aea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perel A. Automated assessment of fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2008;106:1031–1033. doi: 10.1213/ane.0b013e318167abe5. [DOI] [PubMed] [Google Scholar]
- 29.Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Jr, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannesson M, Slieker J, Desebbe O, et al. The ability of a novel algorithm for automatic estimation of the respiratory variations in arterial pulse pressure to monitor fluid responsiveness in the operating room. Anesth Analg. 2008;106:1195–1200. doi: 10.1213/01.ane.0000297291.01615.5c. [DOI] [PubMed] [Google Scholar]
- 31.Derichard A, Robin E, Tavernier B, et al. Automated pulse pressure and stroke volume variations from radial artery: evaluation during major abdominal surgery. Br J Anaesth. 2009;103:678–684. doi: 10.1093/bja/aep267. [DOI] [PubMed] [Google Scholar]
- 32.Michard F, Teboul JL, Richard C. Influence of tidal volume on stroke volume variation. Does it really matter? Intensive Care Med. 2003;29:1613. doi: 10.1007/s00134-003-1886-9. [DOI] [PubMed] [Google Scholar]
- 33.Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102:1511–1517. doi: 10.1213/01.ane.0000209015.21418.f4. [DOI] [PubMed] [Google Scholar]
- 34.Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111:1180–1192. doi: 10.1213/ANE.0b013e3181f08a5b. [DOI] [PubMed] [Google Scholar]