Figure 5.
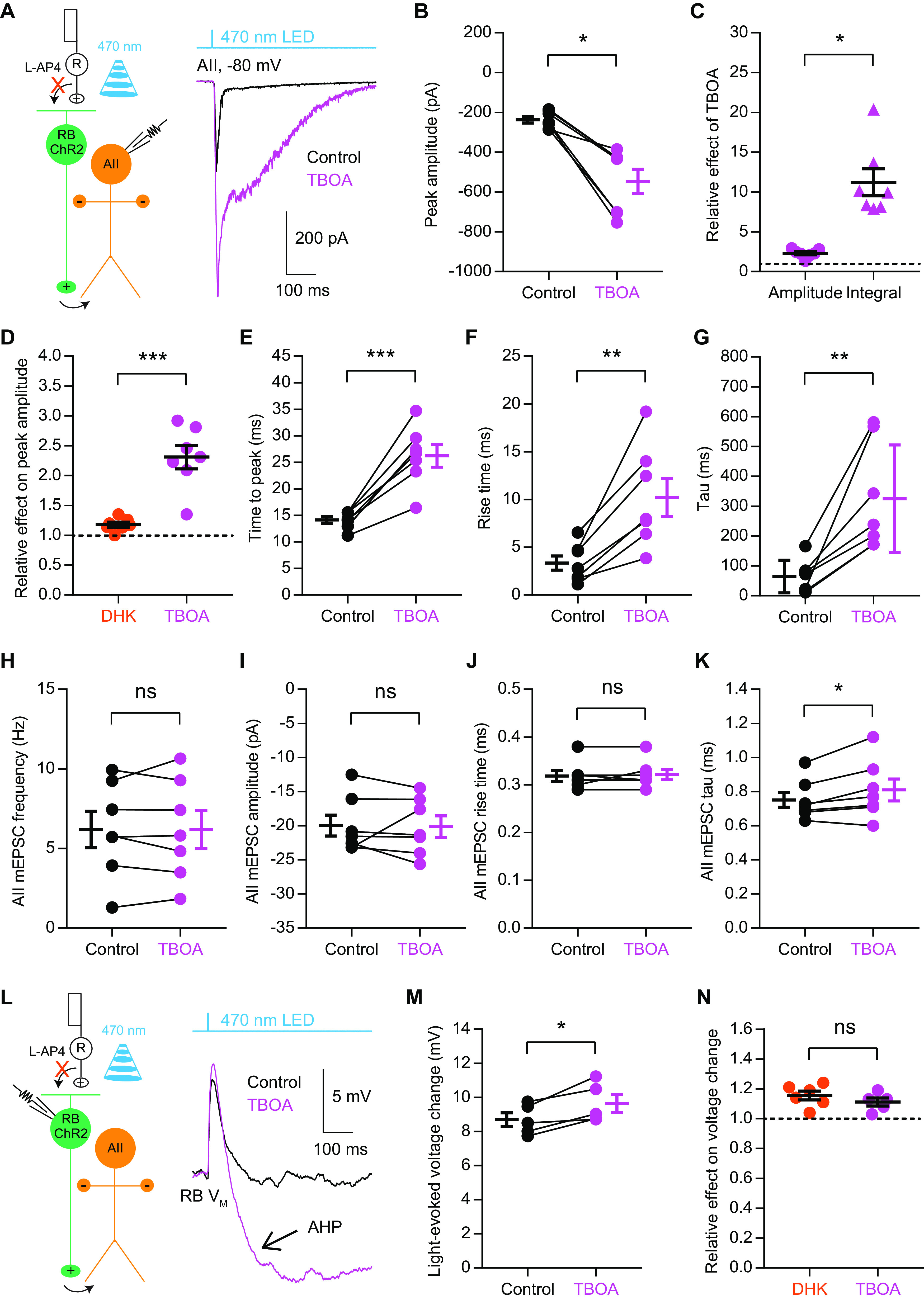
Pharmacological blockade of all EAATs has a significant effect on signal transmission at RB→AII synapses. A, The EPSCs recorded in AII amacrine cells, which were evoked by activating ChR2+ RBs with 470-nm LED light stimulation, were enhanced by 50 μm TBOA, a nonselective blocker of all EAATs. Vhold = −80 mV. R, rod; RB, rod bipolar cell; ChR2, channelrhodopsin-2. B, TBOA increased the peak amplitude of ChR2-evoked EPSCs (n = 7). C, The relative effects of TBOA on the peak amplitude and current integral of AII EPSCs (n = 7). The peak amplitudes/integrals were normalized to the peak amplitude/integral under control condition in each cell before averaging across cells. D, Comparison of the relative effects of DHK (n = 7), a selective EAAT2 blocker, and TBOA (n = 7) on the peak amplitude of AII EPSCs. E–G, TBOA changed the time to peak, rise time and tau of EPSCs (n = 7). H–K, TBOA did not influence the frequency, amplitude, or rise time of mEPSCs recorded in AIIs while increasing the tau slightly (n = 7). mEPSCs, miniature EPSCs. L, The voltage changes in ChR2+ RBs, which were evoked by brief flashes of 470-nm LED light, were increased by 50 μm TBOA. Note that, in the presence of TBOA, a large, long-lasting AHP (arrow) could be recorded in each RB following the light-evoked depolarization. M, TBOA increased the initial voltage changes in RBs evoked by light flashes (n = 5). N, Comparison of the relative effects of DHK (n = 6) and TBOA (n = 5) on light-evoked voltage changes in RBs. The data were represented as mean ± SEM. Wilcoxon signed-rank test or Student’s t test was used where appropriate. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significantly different. See also Table 5.
