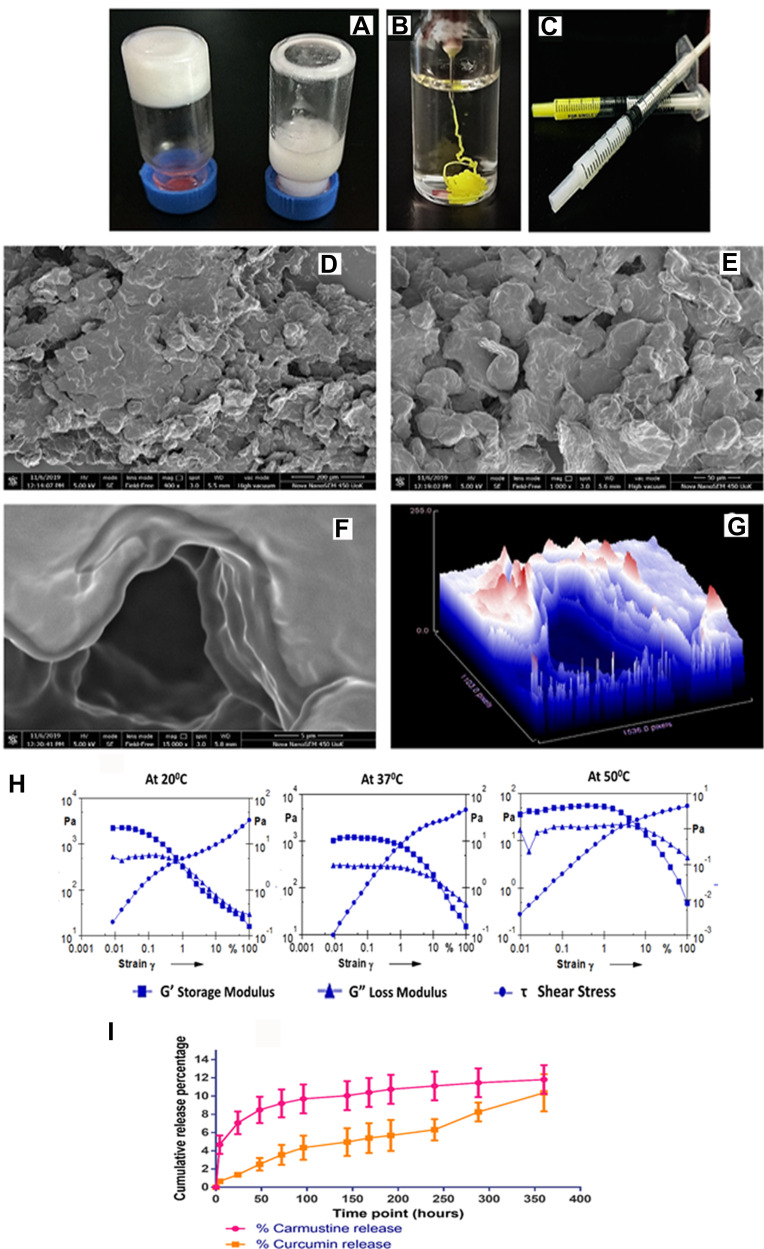
Figure 2.
(A) 20% (w/v) PCL-PEG co-polymer forming hydrogel vs 20% (w/v) monomer PCL diol and PEG diacid blend, that does not form hydrogel. (B) Drug entrapped hydrogel holds its stable gel nature upon injection in excess PBS. (C) PCL-PEG hydrogel can be filled in syringes and ready for application for ease of administration. (D and E) Scanning electron micrograph of surface topology of PCL-PEG (400x) and 1000x, respectively. (F and G) 15,000x magnification of the surface shows layering of polymer in the gel and surface topography graph by Image J analysis of the same. (H) Rheology graph of amplitude sweep at 20°C , 37°C and 50°C with varying strain (0.001–100%). (I) Release study of carmustine and curcumin from the PCL-PEG hydrogel at 37°C in PBS for 360 h.