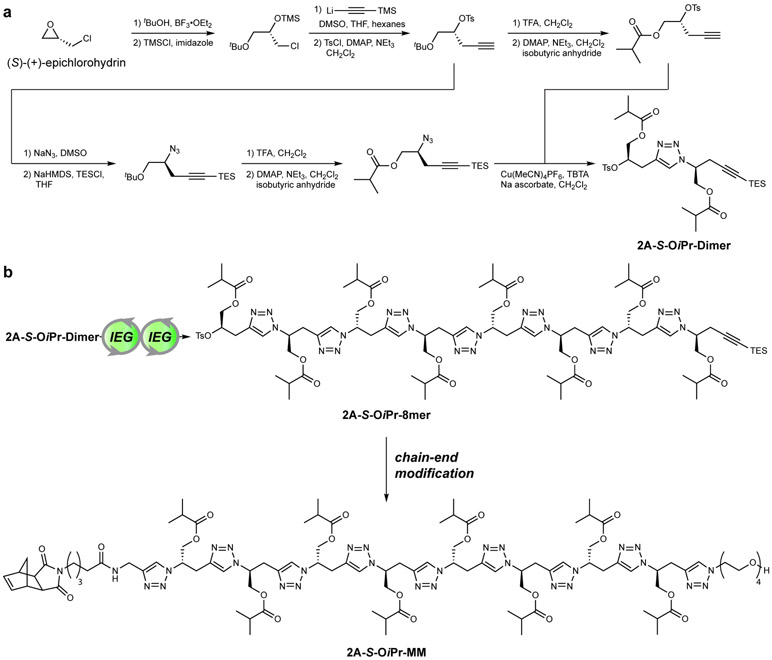
Figure 2. Scheme for “2 atom” iterative exponential growth (IEG) synthesis.
a. Commercially available (S)-(+)-epichlorohydrin is converted in 11 total steps, 9 longest-linear sequence, to IEG dimer 2A-S-OiPr-Dimer (6% yield on the 9 g scale). See Supplemental Information Section C for full details. Note: The “S” label in 2A-S-OiPr-Dimer refers to the source of the two stereogenic centers in this molecule rather than their actual labels based on Cahn-Ingold-Prelog priority rules, the latter of which would lead to one R and one S assignment. b. 2A-S-OiPr-Dimer is subjected to two iterative exponential growth (IEG) cycles, consisting of divergent azidation and alkyne deprotection then convergent copper-catalyzed azide-alkyne cycloaddition (CuAAC), to afford octamer 2A-S-OiPr-8mer. 2A-S-OiPr-8mer is subsequently converted to macromonomer (MM) 2A-S-OiPr-MM for ring-opening metathesis polymerization (ROMP) through coupling of an exo-norbornene derivative (left) and tetraethylene glycol (right) onto the chain ends. Note: due to the use of SN2 reactions that invert the chain end stereochemistry, all of the stereogenic centers in 2A-S-OiPr-MM are S.