Abstract
Purpose
Although children with autism spectrum disorder (ASD) often display motor deficits, the nature of these motor deficits remains unspecified. The purpose of this study was to establish a robust motor profile in children with ASD across a wider range of motor skills by using two professionally administered standardized motor assessments alongside a parent report measure to capture a comprehensive view of motor performance compared to a group of neurotypical peers.
Methods
Complex motor skills, balance and global motor performance were compared in twenty-four children, between the ages of 5–12 years, split into two groups: ASD and typically developing. The Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT-2) and the Movement Assessment Battery for Children, Second Edition (MABC-2) were used to examine skill performance. Motor proficiency was also collected using the parent/caregiver form of the Vineland Adaptive Behavior Scales, 3rd edition (Vineland-3).
Results
Children with ASD presented with significant differences in complex motor skills, balance skills, and global motor performance when compared to their neurotypical peers across all three measures.
Conclusion
This preliminary study indicated that the children with ASD had greater difficulty with global motor performance, including more difficulty performing complex motor tasks and balance tasks compared to their neurotypical peers. The parents of the children with ASD reported decreased proficiency of motor skills. Overall, the children with ASD demonstrated deficits performing tasks that targeted strength, speed, agility, coordination and both static and dynamic balance. While manifestations of motor skill deficits specific to the ASD population are variable, physical therapists should be included in the ongoing assessment and implementation of comprehensive therapeutic plans for children with ASD.
Keywords: autism spectrum disorder, motor skills, motor proficiency, balance, coordination
Introduction
Although it’s widely accepted that motor deficits are a pervasive feature among children with autism spectrum disorder (ASD; Fournier et al. 2010), impacting as many as 79% of children with ASD (Green et al. 2009), and potentially serving as an early diagnostic indicator (May et al. 2016), a clear understanding of the underlying motor profile of children with ASD remains elusive. In children, motor impairments are related to physical activity levels, social play, social responsiveness, cognitive development and academic success (Gladfelter et al. 2020, Hannant et al. 2018, Lang et al. 2010, Memari et al. 2013, Kenny et al. 2016, Pan 2009, Holloway et al. 2018). In children with ASD, reduced social play and difficulties attending to their environment and peers can also limit future opportunities to develop higher level motor skills (Kenny et al. 2016, Pan 2009, Holloway et al. 2018). This intertwining influence of motor skills across nearly all aspects of a child with ASD’s life points to a dire need to better understand the motor characteristics of ASD. To facilitate increasing opportunities for interactions with their environment and with their peers and to enhance their overall quality of life, a more complete picture of the motor impairments in children with ASD must be identified and addressed.
One of the challenges of identifying specific motor deficits in ASD is that they manifest differently as children grow. In early development, acquisition of gross motor skills requires both strength and sensory feedback to learn how to move against gravity and active practice. As infants begin to crawl, walk, run, and jump, the physical demands of motor skills change. For clinicians working with children with ASD, recognizing these early changing motor behaviors in ASD is critical for early diagnosis and intervention. A recent narrative review by May et al. (2016), concluded that motor impairment may be an early diagnostic sign or behavioral marker in ASD. Poor postural control in infants, as evidenced by head lag, has been associated with a subsequent diagnosis of ASD (Flanagan et al. 2012). In fact, poor postural control and gross motor delays in children with ASD could indicate possible neurodevelopmental disruption in these children (e.g. Downey et al. 2012, Ozonoff et al. 2008). Postural control requires an infant or toddler to be able to produce a motor response to stay upright (e.g. sitting, standing, or walking) prior to something that would cause them to lose their balance (anticipatory motor responses) (Hadder-Algra 2018). Developmentally, postural control deficits lead to delays in mobility, including crawling and walking. Once children are upright and walking, the effects of gravity and reduced postural stability will present as balance deficits.
Balance (or postural stability) is the ability to maintain your center of mass over your base of support (Kloos et al. 2018). The ability to maintain postural control is a component of balance. Balance control involves the processing of sensory information, timing and sequencing of muscle movements and motor planning (Kloos et al. 2018). Because children with ASD show poor postural control as infants (Flanagan et al. 2012), it is perhaps unsurprising that they continue to show reduced postural stability as they grow, especially when somatosensory input is disrupted (Minshew et al. 2004). Impaired body awareness and perception impact postural righting and equilibrium for dynamic balance control in children with ASD (Wang et al. 2016). Even in children with ASD between the ages of 7 and 18 impairments in both static and dynamic stance are evident on force platform measures of postural orientation and sway (Wang et al. 2016). In fact, the presence of static balance deficits in children with ASD is well-supported in the literature (Minshew et al. 2004, Whyatt et al. 2012, Ament et al. 2015), but dynamic balance deficit findings are mixed. For example, Whyatt et al. (2012) compared the performance of 7- to 10-year old children with ASD and non-verbal IQ matched peers on individual items of the Movement Assessment Battery for Children-2nd edition (MABC-2; Henderson et al. 2015), a standardized test of motor performance in children. They uncovered deficits with catching a ball and static balance (e.g. single leg stance) in children with ASD, but not with dynamic balance (e.g. jumping or hopping) tasks. However, Pan (2014), reported a wider range of motor skill deficits, including dynamic balance, in adolescents (ages 10–17) with ASD as measured by Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2; Bruininks et al. 2005). The convergent validity of the MABC-2 and BOT-2 has been challenged in the 7–10-year-old age group (Lane et al. 2015), and the conflicting reports in dynamic balance skills may be due to the variability in the assessment tools. However, there are no studies to date that directly compare performance of motor skills of children with ASD and neurotypical peers across both the MABC-2 and BOT-2.
Balance difficulties are not the only motor factor that have been identified in ASD. In some reports, children with ASD show particular deficits on complex motor tasks (Whyatt et al. 2012, Paquet et al. 2019). Deficits in complex motor skills, such as skipping or dribbling a ball, are more specifically defined as intricate body actions with finer control and coordination of multiple body parts (Whyatt et al. 2012, Kraft et al. 2015). Furthermore, more global motor deficits have been implicated by others (Green et al. 2009, Pan 2009, Pan 2014). A global motor deficit refers to a more widespread delay in multiple motor areas (Tükel et al. 2015). For example, children would demonstrate widespread issues with both fine and gross motor skills. This wide variety of motor deficit findings in the ASD literature is likely because most studies look at motor skills in isolation; less research has taken a comprehensive look at the full motor profile of children with ASD. Without a comprehensive motor profile of ASD, it’s difficult for clinicians to know which motor features could be used to promote earlier identification and awareness in parents.
Parent perceptions of their child’s social communication tendencies are often the primary concern when seeking a diagnosis for ASD (Yimyang et al. 2017). However, motor concerns can be raised possibly two years prior to social cues. This could lead to earlier diagnosis and interventions of ASD. Leonard et al. (2015), suggested that infant gross motor delays predicted expressive language delays in toddlers later diagnosed with ASD. Other studies have reported head lag in infants as young as 6 months old (Flanagan et al. 2012) and disrupted or delayed gestures are evident as early as nine months of age (Gordon et al. 2015, Veness et al. 2012). But parents who report motor skills as their earliest concern only make up approximately 23% of cases (Guinchat et al. 2012). Even though parent perceptions of social skills have been linked to their child’s motor performance (e.g. Gladfelter et al. 2020, Hirata et al. 2015), the motor deficits associated with ASD are not leading as many parents to seek treatment or a diagnosis. Parent report measures of motor skills are widely available and have been considered reliable and valid measures for children with ASD (Balboni et al. 2016). For example, the Vineland Adaptive Behavior Scales, 3rd edition parent/caregiver form (Vineland-3; Sparrow et al. 2016) is commonly used to support the diagnosis of intellectual or developmental disabilities. The Vineland-3 is one of the most widely used tools that can assess these daily living skills, or adaptive behavior, and verify possible correlations between development and adaptive abilities (La Malfa et al. 2009). The Vineland-3 included four domains: communication, daily living skills, socialization, and motor skills. Because of its use in conceptualizing developmental delays, reliability, and validity measurements of adaptive behavior (Farmer et al. 2020), including global motor skills, the Vineland-3 could appropriately be used to help elucidate the motor deficits observed directly by parents of children with ASD.
In summary, although a growing body of evidence indicates that motor deficits are a common characteristic of children with ASD, and that these differences emerge early in development, there is conflicting evidence on which aspects of motor performance (static balance, dynamic balance, complex or global) are impaired as compared to neurotypical peers. Past challenges of assessing motor behaviors in isolation, relying on a single motor performance tool, and an absence of included parent observations of motor behavior beyond the laboratory setting have all been obstacles to identifying a more comprehensive motor profile in children with ASD. As such, the purpose of this preliminary study was to establish a robust motor profile in children with ASD across a wider range of motor skills by using two full-scale, standardized motor assessments (MABC-2 and BOT-2) alongside a parent questionnaire (Vineland-3) to capture a comprehensive view of motor performance, and then compare the performances to their neurotypical peers.
Materials and methods
Participants
Twenty-four children participated in this study. Twelve participants with ASD (M = 8.71 years, range 5–12 years, 11 male) and twelve typically developing (TD) peers (M = 8.74, range 5–11 years, 10 male) were assessed for motor performance. According to a power analysis using G*Power statistical software (Buchner et al. 2017, Faul et al. 2007), a total sample size of 18 participants would be sufficient with an alpha level of .05, power of .80, and a moderate effect size of .25 for a planned 2 group between factors (ASD vs. TD) and 10 factor within (MABC-2, Vineland-3 and BOT-2 total and subscale scores) multivariate analysis of variance (MANOVA) as the set parameters for a conducting a between group comparison. In accordance with the recruitment and implementation procedures pre-approved by the university’s Institutional Review Board, all participants were recruited through flyers to local schools, an ASD caregiver group, a local NPR advertisement, and by word of mouth. Written parental consent was obtained from a parent or legal guardian while assent from the child was obtained verbally prior to beginning data collection.
To be included in the group with ASD, the children must have obtained a prior medical diagnosis of ASD by a physician. All ASD diagnoses were confirmed by the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al. 2012) and the Childhood Autism Rating Scale, Second Edition (CARS-2; Schopler et al. 2010), as administered by a trained graduate speech-language pathology research assistant.
A neurotypical control group was included to more strictly control for variation in motor performance that could be attributed to local geographic or demographic factors that may not be as well reflected in the norming sample of the selected standardized tests. The CARS-2 was administered to rule out a possible ASD diagnosis in the children with TD.
All children were English-speaking and were able to complete the motor assessments without need for modification of the instructions. Once included in the study, the parents completed the Social Responsiveness Scale, Second Edition (SRS-2), a parent questionnaire with excellent correspondence to the ADOS-2 (Duvekot et al., 2015). Higher scores on the SRS-2 reflect more impaired ability to respond to social needs. The Test of Nonverbal Intelligence, Third Edition (TONI-3; Brown et al. 1997) was completed for all ASD participants and eight of the twelve TD participants, due to participant time constraints and prioritized completion of motor assessments. None of the parents reported a history of or concerns for intellectual disabilities for any of the participants who were not given the TONI-3. Both groups yielded similar nonverbal intelligence scores (MeanASD = 105.170; MeanTD = 109.380; p = 0.440). A summary of participant characteristics is listed in Table 1.
Table 1.
Participant demographic characteristics
| Demographic data | ASD Mean (SD) | Range | TD Mean (SD) | Range |
|---|---|---|---|---|
| Agea (years) | 8.71 (1.69) | 5–12 | 8.74 (2.42) | 5–11 |
| CARS, raw score | 27.62 (3.97) | 20.5–34.5 | 15.30 (0.48) | 15–16 |
| SRS-2, T-score | 74.92 (10.28) | 61–90 | 45.75 (5.48) | 36–55 |
| ADOSb, symptom severity | 8.03 (1.49) | 7–12 | n/a | n/a |
| TONI3c, standard score | 105.17 (13.12) | 78–125 | 109.38 (14.98) | 78–137 |
| Vineland-3 Communication | 78.80 (8.74) | 61–91 | 101.00 (14.94) | 74–117 |
| Vineland-3 Socialization | 70.70 (5.08) | 44–96 | 100.55 (13.28) | 78–120 |
| Vineland- Daily living skills | 76.70 (12.00) | 48–96 | 98.09 (16.10) | 74–127 |
Note: ASD = Autism spectrum disorders; TD = typical development; SD = standard deviation; CARS = Childhood Autism Rating Scale; SRS = Social Responsiveness Scale; ADOS = Autism Diagnostic Observation Scale, 2nd edition; TONI3 = Test of Non-verbal Intelligence, 3rd edition; n/a = not applicable.
N = 12 for each group;
N = 12 for ASD group only;
N = 12 for ASD group, N = 8 for TD group.
Instrumentation and data collection procedures
To comprehensively assess the static and dynamic balance, complex motor skills, and global motor performance of children with ASD and their peers, the principal investigator, a physical therapist with board certified pediatric specialty, and two trained graduate research assistants, administered two standardized assessments of motor skills for children, the MABC-2 (Henderson et al. 2015) and the BOT-2 (Bruininks et al. 2005), as instructed in the administration manuals. Additionally, the Vineland Adaptive Behavioral Scales, third edition (Vineland-3; Sparrow et al. 2016) was given to the parents of the participants as a measure of global motor performance. To accommodate the needs of the families, five of the children in the ASD group and seven in the TD group completed both motor assessments within the same day. The remaining children in each group completed both motor assessments across two sessions, which occurred on average 12 days apart (range 1 day to 1 month). To be consistent across all participants, the MABC-2 was always administered first, followed by the BOT-2. The entirety of each assessment tool was always completed within one session.
Movement assessment battery for children, 2nd edition (MABC-2)
The MABC-2 consists of a total of 24 motor tasks, divided into 3 age bands, namely Band 1 (ages 3–6), Band 2 (ages 7–10) and Band 3 (ages 11–16). Each age band consists of a total of 8 subtest items that progressively are more difficult in the subsequent age bands. The subtest items are divided into three components: Manual Dexterity, Aiming and Catching, and Balance. The MABC-2 combines the scores in the three component categories (manual dexterity, aiming/catching and balance) into one total MABC-2 standard score. The investigators converted the raw and component scores for each item and subtest to standard scores using age-matched normative data to analyze the MABC-2 data in accordance with the instructions in the administration manual (Henderson et al. 2015). For MABC-2, the mean standard score is 10 with a standard deviation of 3. According to the manual, a motor performance with a standard score of 5 or less is indicative of significant movement difficulty (Henderson et al. 2015).
Bruininks-Oseretsky test of motor proficiency, 2nd edition (BOT-2)
The investigators then completed the following subtests on the BOT-2 (see Table 2): Manual Dexterity, Upper Limb Coordination, Balance, Bilateral Coordination, Strength, and Speed and Agility. Raw scores were converted to scaled scores based on age and gender matched normative data (Bruininks et al. 2005). The BOT-2 combines subtest scale scores into composite standard scores (see Table 2). The balance and bilateral coordination scaled subtest scores combined to convert to the body coordination composite standard score, while the speed and agility and strength subtests combined to form the strength and agility composite standard score. The sum of the balance, bilateral coordination, speed and agility, and strength scaled scores was used to calculate the gross motor composite standard score. The manual dexterity and upper limb coordination subtests were also completed on the BOT-2 in order to be comparable to the manual dexterity and aiming/catching completed in the MABC-2, respectively. For the BOT-2, the mean for a subtest scale score is 15 with a standard deviation of 5; the mean for a composite standard score is 50 with a standard deviation of 10.
Table 2.
Bruininks-Oseretsky test of motor proficiency, 2nd edition categorization of subtests and motor composites
| Subtest (scale scores yielded) | Motor-area composite (standard scores yielded) | Motor composite (standard scores yielded) |
|---|---|---|
| 1. Fine motor precision | Fine manual control (not completed) | Fine motor composite |
| 2. Fine motor integration | ||
| 3. Manual dexterity (5 items) | Manual coordination (completed) | |
| 7. Upper-limb coordination (7 items) | ||
| 4. Bilateral coordination (7 items) | Body coordination (completed) | Gross motor composite |
| 5. Balance (9 items) | ||
| 6. Running speed and agility (5 items) | Strength and agility (completed) | |
| 8. Strength (5 items) |
Note: Shaded subtests were completed to yield composite scores.
Vineland adaptive behavioral scales, third edition (Vineland-3)
Parents completed the Vineland Adaptive Behavioral Scales, Third edition (Vineland-3; Sparrow et al. 2016) Parent/Caregiver form. If the children were 10 or older, the Vineland-3 is unable to yield a standard score for the motor domain. Therefore, ASD and TD group n = 9 for the Vineland-3 motor domain. All other portions of the Vineland-3 were completed by twelve parents in the TD group and nine parents in the ASD group. Investigators utilized the adaptive behavior composite (total score for all domains) as well as the motor skills domain score (sum of gross motor and fine motor subtests) for a global motor score from the parent’s perspective.
Balance, complex motor, and global motor performance
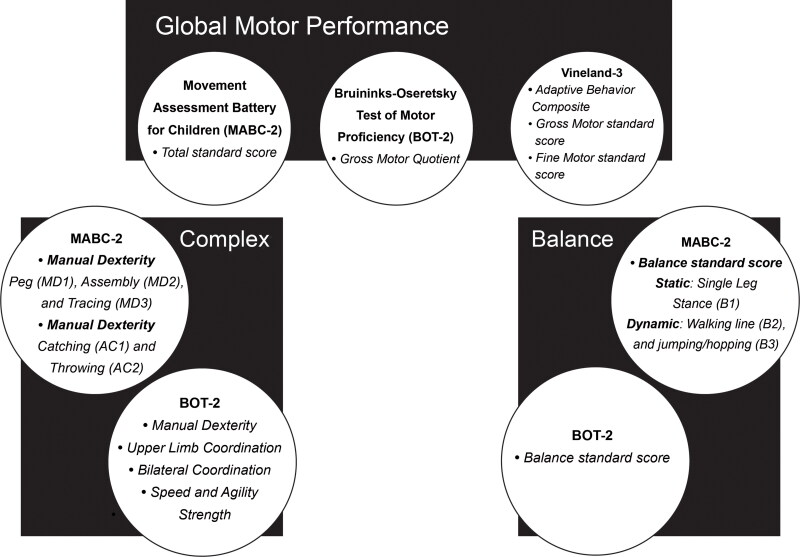
To better understand which areas of motor skills are implicated in children with ASD, specific subtests and individual test items were designated as reflecting either balance skills, complex motor skills, or global motor performance. To capture the balance skills of participants the BOT-2 and MABC-2 subtests were compared, and the MABC-2 items compared static and dynamic balance tasks. For complex motor skills, the following BOT-2 subtests were compared: manual dexterity, upper limb coordination, bilateral coordination, speed and agility and strength. On the MABC-2, the following subtests and their scaled item scores were compared: manual dexterity and aiming and catching. Finally, the MABC-2 total test score, BOT-2 gross motor quotient (GMQ) and Vineland-3 were used to reflect global motor performance in the children with ASD and TD. Figure 1 depicts each motor category and its test item components.
Figure 1.
Motor subtests and items categorized for analysis
Inter-rater reliability procedures
Inter-rater reliability procedures were completed in three stages: 1) watching a BOT-2 (Bruininks et al. 2005) training DVD and reviewing the examiner books with live instructional training by the lead investigator for both the MABC-2 (Henderson et al. 2015) and the BOT-2 (Bruininks et al. 2005) assessments, 2) the graduate-level researchers studying physical therapy observed the lead investigator, a board certified specialist in pediatric physical therapy, and concurrently assessed and scored motor assessments of ten children who agreed to participate but were not eligible for this study, and 3) the investigators used consensus building until 100% agreement was achieved on all items. In addition, all motor assessments were videotaped and used for reliability checks and subsequent consensus building. While training, the investigators obtained consensus for all item performance scores and subsequent calculation of the standard and composite scores.
During data collection for this study, the lead investigator completed live (concurrent) assessments with a graduate student investigator as well as reliability checks on 16 of the 24 participants’ motor assessments. The graduate student investigators completed the remaining eight assessments. The lead investigator completed additional reliability checks using video recordings in four of those remaining eight participants’ assessments. In this study, two-way mixed effects, consistency, single rater/measurement intraclass coefficient correlation (ICC) was adequate, as a clinical measure, for the subtests of the MABC-2 (ICC (3, 1) = 0.988–0.994) and BOT-2 (ICC (3, 1) = 0.970–0.996).
Statistical analysis
The raw scores for both the MABC-2 and the BOT-2 were converted to scaled or standard scores using age-specific norms, according to their age at the time of each evaluation. Investigators only utilized the scaled or standard scores for data analysis. SPSS v.25 (IBM Corp., Armonk, New York) was used to complete the statistical analyses. The data met Levene’s test for homogeneity of variance. The ASD/TD groups were entered into a MANOVA to compare the mean motor performance as follows: 1) the MABC-2 and Vineland-3 total test scores and BOT-2 gross motor quotient were compared for global motor performance, 2) BOT-2 manual dexterity, upper limb coordination, and speed and agility, and MABC-2 manual dexterity and aiming and catching were compared to address complex motor actions, and 3) BOT-2 and MABC-2 subtest balance scores were compared to address global balance, and the MABC-2 individual task standard scores were used to compare static and dynamic balance performance separately. Even though total test scores on the MABC-2 are considered to have stronger psychometric properties (Griffiths et al. 2018, Henderson et al. 2015), to more closely relate these results to those previously reported by Whyatt et al. (2012), MABC-2 individual item standard scores were also explored. An alpha level was set to 0.050 for all statistical analyses.
Results
Between the ASD and TD groups, a significant interaction effect was found for motor performance on the MABC-2, F (1, 22) = 9.988, p < .001; Wilk’s Λ = 0.322, partial η2 = .678, and BOT-2, F (1, 22) = 4.712, p = .007; Wilk’s Λ = 0.203, partial η2 = .797. However, noted in each section below, the ranges of standard scores for ASD and TD participants overlapped. It is important to not some participants with ASD outperformed participants in the TD group. Specific results based on dependent variables, balance, complex motor performance, and global motor performance, are summarized below.
Static and dynamic balance
There was a statistically significant difference between the ASD and TD groups in overall balance performance on both the MABC-2 and the BOT-2. On the MABC-2, the ASD group standard scores ranged from 2 to 9, and the TD group ranged from 5 to 15. On the BOT-2, the balance subtest scaled scores ranged from 5 to 13 (ASD) and 8 to 19 (TD). While we continue to see an overlap in skill performance, the overall balance skill performance for the ASD group was significantly lower than the TD group on the MABC-2, F (1, 22) = 20.106, p < .001, partial η2 = .478, and BOT-2, F (1, 22) = 14.457, p = .001, partial η2 = .408. In addition, there were mixed results for both static and dynamic balance tasks when comparing MABC-2 item analysis of the ASD and TD groups. All balance results, including means and standard deviations, are summarized in Table 3.
Table 3.
Comparison of balance motor performance
| Test: subtest, task | ASD group (n = 12) |
Typical group (n = 12) |
F | p-value | Partial Eta squared | ||
|---|---|---|---|---|---|---|---|
| SS | SD | SS | SD | ||||
| BOT-2: overall balance | 9.090 | 2.700 | 14.000 | 3.411 | 14.457 | 0.001* | 0.408 |
| MABC-2: overall balance | 5.640** | 1.859 | 9.830** | 2.758 | 20.106 | 0.000* | 0.478 |
| MABC-2: Balance, static B1 | 5.417 | 2.109 | 9.333 | 2.708 | 15.626 | 0.001* | 0.415 |
| MABC-2: Balance, dynamic B2 | 8.083 | 4.055 | 10.417 | 2.875 | 2.644 | 0.118 | 0.107 |
| MABC-2: Balance, dynamic B3 | 6.083 | 3.117 | 10.000 | 2.558 | 11.318 | 0.003* | 0.340 |
Note: ASD = Autism spectrum disorders; TD = typical development; SS = mean scaled score, SD = mean standard deviation, SEM = standard error of the mean; BOT-2 = Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition; MABC-2 = Movement Assessment Battery for Children, 2nd edition; B1 = single leg stance; B2 = walking line; B3 = jumping/hopping.
significant at < 0.05;
Standard score.
MABC-2 subtest item analysis: MANOVA
The mean standard scores and univariate F tests (see Table 3) revealed the TD group performance was better than the ASD group with respect to static Balance Skills, B1 (single leg stance), F (1, 22) = 15.626, p = .001, partial η2 = .415. However, there were mixed results in relation to dynamic Balance Skills. Performance in walking on a line (B3) revealed the TD group outperformed the ASD group significantly, F (1, 22) = 11.318, p = .003, partial η2 = .340, but jumping/hopping (B2) performance was not significantly different. Accordingly, one item in each of the MABC-2 subtests was not significantly different between the ASD and TD group performance.
Complex motor skills
The ASD participant standard scores for MABC-2 manual dexterity ranged from 2 to 10, while TD participant scores ranged from 3 to 12. The MABC-2 standard scores for the ASD group were significantly lower than the TD group, in manual dexterity performance on both the MABC-2, F (1, 22) = 5.996, p = .023, partial η2 = .214, and BOT-2, F (1, 22) = 13.222, p = .002, partial η2 = .386.
On the BOT-2, the bilateral coordination, upper limb coordination, strength, and speed and agility scaled scores for the ASD group were significantly lower than the TD group. ASD BOT-2 scaled scores ranged from 6 to 12 in bilateral coordination, while the TD range was 9 to 24, F (1, 22) = 9.678, p = .005, partial η2 = .31, and upper limb coordination scaled scores ranged from 6 to 15 in the ASD group and 9 to 24 in the TD group, F (1, 22) = 18.859, p < .001, partial η2 = .473. ASD group BOT-2 scaled scores ranged from 6 to 12 in strength, while the TD group range was 8 to 24, F (1, 22) = 27.335, p < .000, partial η2 = .566, and from 5 to 18 (ASD) and 9 to 23 (TD) in speed and agility, F (1, 22) = 11.039, p = .003, partial η2 = .345. However, there was not a statistically significant difference between the ASD and TD groups in aiming and catching performance on the MABC-2, F (1, 22) = 3.708, p = .067, partial η2 = .144. A summary of all complex motor task results, including means and standard deviations, are presented in Table 4.
Table 4.
Comparison of complex motor task performance
| Test: subtest | ASD group (n = 12) |
Typical group (n = 12) |
F | p-value | Partial Eta squared | ||
|---|---|---|---|---|---|---|---|
| SS | SD | SS | SD | ||||
| BOT-2: Manual dexterity | 8.730 | 3.636 | 14.830 | 4.345 | 13.222 | 0.002* | 0.386 |
| BOT-2: Upper limb coordination | 8.910 | 2.737 | 15.920 | 4.660 | 18.859 | 0.000* | 0.473 |
| BOT-2: Bilateral coordination | 10.180 | 2.523 | 15.250 | 4.827 | 9.687 | 0.005* | 0.315 |
| BOT-2: Speed/agility | 11.180 | 3.763 | 16.330 | 4.010 | 11.039 | 0.003* | 0.345 |
| BOT-2: Strength |
8.640 |
1.690 |
16.730 |
4.599 |
27.335 |
0.000* |
0.566 |
| Test: subtest (task) |
Std score |
SD |
Std score |
SD |
F
|
p-value |
Partial Eta squared |
| MABC-2: Manual dexterity | 5.000 | 2.730 | 7.508 | 2.429 | 5.996 | 0.023* | 0.214 |
| MABC-2: MD1 | 6.583** | 2.644 | 9.250** | 2.667 | 6.046 | 0.022* | 0.216 |
| MABC-2: MD2 | 6.000** | 3.384 | 8.917** | 2.746 | 5.375 | 0.030* | 0.196 |
| MABC-2: MD3 | 3.750** | 3.571 | 5.667** | 3.846 | 1.601 | 0.219 | 0.068 |
| MABC-2: Aiming & catching | 6.250 | 2.734 | 8.750 | 3.571 | 3.708 | 0.067 | 0.144 |
| Mabc-2: Ac1 | 7.000** | 2.174 | 10.083** | 3.147 | 7.799 | 0.011* | 0.262 |
| MABC-2: AC2 | 6.583** | 3.118 | 6.583** | 2.610 | 0.000 | 1.000 | 0.000 |
Note: ASD = Autism spectrum disorders; TD = typical development; SS = mean scaled score, Std Score = mean standard score, SD = standard deviation, SEM = standard error of the mean; BOT-2 = Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition; MABC-2 = Movement Assessment Battery for Children, 2nd edition; MD1 = pegboard item; MD2 = assembly item; MD3 = tracing item; AC1= catching; AC2 = throwing.
significant at < 0.05;
Scaled score.
MABC-2 subtest item analysis: MANOVA
The multivariate result was significant for ASD/TD group, Pillai’s Trace = .624, F (1, 22) = 3.116, p = .028, indicating a significant main effect for the combined MABC-2 dependent variables (Manual Dexterity (MD) subtest items 1, 2, 3; Ball Skills (AC) subtest items 1, 2; Balance (B) subtest items 1, 2, 3) and group (ASD and TD). When the subtest items were analyzed separately, the univariate F tests revealed the item mean standard scores (see Table 4) of the ASD group were significantly lower than TD group performance with respect to Manual Dexterity skills for both MD1 (peg-board), F (1, 22) = 6.049, p = .022, partial η2 = .216, and MD2 (assembly), F (1, 22) = 5.375, p = .030, partial η2 = .196, as well as Ball Skills for AC1 (catching), F (1, 22) = 7.799, p = .0110, partial η2 = .262. However, the ASD and TD groups performed similarly for MD3 (tracing) and AC2 (throwing) items. Accordingly, one item in each of the subtests was not significantly different in performance on the MABC-2.
Global motor performance
MABC-2 component scores were combined for an overall or total test standard score. The ASD participant total test standard scores ranged from 1 to 8, while TD participant scores ranged from 4 to 13. The ASD total MABC-2 scores were significantly lower than the TD group, F (1,22) = 11.203, p = 0.003, partial η2 = .337. BOT-2 subtests are combined for composite scores and a gross motor quotient was calculated for each child. The range of BOT-2 gross motor quotients for the TD group was 36–67, while the range for the ASD group was 31–42. The comparison of the BOT-2 three composite scores and gross motor quotient also yielded significant results: manual coordination, F (1, 22) = 23.303, p = .000, partial η2 = .526, body coordination, F (1, 22) = 28.012, p = .000, partial η2 = .572, strength and agility, F (1, 22) = 21.830, p < .001, partial η2 = .510, and gross motor quotient, F (1, 22) = 25.059, p < .001, partial η2 = .544.
Similar to that of the MABC-2 and BOT-2 motor performance, there was a statistically significant difference in the multivariate result on the Vineland-3. The ASD mean scores were significantly lower than the TD group for motor skill proficiency and overall adaptive behavior on the Vineland-3 (F (1, 15) = 5.796, p = .008; Wilk’s Λ = .341, partial η2 = .659). Mean scores for gross motor, fine motor, overall motor and overall adaptive behavior are listed in Table 5. There was a statistically significant difference in each area: gross motor (F (1, 15) = 17.599, p = .001, partial η2 = .540), fine motor (F (1, 15) = 18.109, p = .001, partial η2 = .547), overall motor score (F (1, 15) = 27.331, p = .001, partial η2 = .646), and overall adaptive behavior score (F (1, 15) = 14.882, p = .001, partial η2 = .498).
Table 5.
Comparison of global motor performance
| Test: subtest | ASD group (n = 12) |
Typical group (n = 12) |
F | p-value | Partial Eta squared | ||
|---|---|---|---|---|---|---|---|
| Std Score | SD | Std Score | SD | ||||
| MABC-2: Total test score | 4.670 | 2.103 | 8.170 | 2.949 | 11.203 | 0.003* | 0.337 |
| BOT-2: Gross motor quotient | 36.909 | 4.085 | 51.500 | 8.827 | 25.059 | 0.000* | 0.544 |
| Vineland-3: Motor domain scorea | 82.220 | 7.823 | 105.880 | 10.763 | 27.331 | 0.000* | 0.646 |
| Vineland-3: ABCa | 76.560 | 7.367 | 93.250 | 10.389 | 14.882 | 0.002* | 0.498 |
Note: ASD = Autism spectrum disorders; TD = typical development; Std Score = mean standard score, SD = mean standard deviation, SEM = standard error of the mean; MABC-2 = Movement Assessment Battery for Children, 2nd edition; BOT-2 = Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition; Vineland-3 = Vineland Adaptive Behavior Scales, 3rd edition; ABC = Adaptive Behavior Composite.
significant at < 0.05.
n = 9 for ASD group and n = 9 for TD group.
Discussion
The purpose of this preliminary study was to establish a comprehensive motor profile in children with ASD across a wide range of motor skills, particularly complex motor tasks, balance, and global motor performance, by using two full-scale, standardized motor assessments alongside a parent questionnaire to capture a comprehensive view of motor performance, and then compare performances to their neurotypical peers. It is important to note that not all children with ASD presented with motor deficits and some of the participants with ASD outperformed participants in the TD group. This is a critical reminder that even in children with known motor impairments there is significant variability in motor performance, and the potential motor impact on daily living skills should be evaluated by a healthcare professional.
As other researchers have reported previously (Green et al. 2009, Pan 2009, Pan 2014), the results of this study indicated that children with ASD demonstrated deficits in global motor skill performance. When performance was assessed at the component or subtest level, motor deficits were revealed in children with ASD as compared to children with TD, not only for balance, but also for manual dexterity, strength, agility, and coordination, including bilateral and upper limb coordination. These results support and expand previous studies indicating more global challenges with motor tasks with both coordination and balance deficits in the ASD population, which may be due to underlying deficits with strength, postural control, and stability (Green et al. 2009, May et al. 2016, Lane et al. 2015, Hannant et al. 2018).
On the balance tasks, the children with ASD demonstrated the most significant discrepancy in both static balance (single leg stance) and one of the two dynamic (jumping/hopping) items. There was no difference in performance of the tandem walking dynamic balance item between the two groups. As was found in the study by Whyatt et al. (2012) comparing performance of children with ASD to a nonverbal IQ control group, the children in our study performed differently on the pegboard (MD1), catching a ball (AC1), and static balance (B1) tasks compared to their typical peers. Contrary to the Whyatt and Craig results, despite participants with similar age and non-verbal IQ scores, the children in the current study also demonstrated difficulty with the jumping/hopping task as compared to their peers. The divergence in findings is likely demonstrative of the variability in ASD motor impairment seen clinically and supports the need for individualized assessments of global motor skills to determine if direct physical therapy services would complement the development and functioning of children with ASD. Importantly, both studies revealed motor weaknesses across a range of tasks that were not exclusive to the area of balance.
When complex motor performance was considered in the subtest item level on the MABC-2 (Tables 3 and 4), children with ASD demonstrated difficulty with both peg-board and assembly items in the manual dexterity component but performed similarly to the children in the TD group on the tracing item. This may be indicative of more difficulty with precision and timing. The tracing was a task of precision that was untimed. This may suggest that when given adequate amount of time (and no pressure of completion within a certain amount of time), the children with ASD may perform comparably to that of their peers. Similarly, in the aiming and catching component, children with ASD had difficulty with catching but not throwing. Throwing certainly requires less anticipatory responses than catching. Perhaps, the deficits are related to feedforward loops and anticipatory responses (Foster et al. 2020, Ganglmayer et al. 2019, Schmitz et al. 2003), which is consistent with theory of mind (Devaine et al. 2014). This could help explain children with ASD demonstrating less desire to participate in unstructured motor activities or during recreational activities when peer actions and ball play can be unpredictable (Solish et al. 2010, Marquis et al. 2015, McCoy et al. 2016).
In clinical practice, it is important to determine which tool you will use during motor assessment of a child. Although significant differences were noted between the ASD and TD groups on all listed subtests, differences were also observed between MABC-2 and BOT-2 scores for the same subtest. For example, no statistical significance was found in the aiming and catching subtest of the MABC-2. With the MABC-2 mean standard score of 10 and standard deviation of 3, both groups scored below average (greater than 1 standard deviation from the mean) for throwing. When this score was combined with catching, the Aiming and Catching score for the children with ASD was still below average but the TD group performance moved into the average range. However, scores on the BOT-2 upper limb coordination subtest, which also assessed throwing and catching as well as bouncing a ball, did reach statistical significance between the two groups with the ASD group. The BOT-2 mean for scaled scores is 15 with a standard deviation of 5; ASD group performance for upper limb coordination was below average (greater than 1 standard deviation below the mean) while the TD group performance was average. The question remains if both tests are standardized, valid and reliable, why would you select one over the other in children with ASD?
There are key differences between the tools and the administration. The MABC-2 has fewer subcomponents and items, which may limit its internal consistency, however it is also noted to have good reliability and predictive validity (Griffiths et al. 2018). In contrast to the MABC-2, the sheer number of items needed to complete the BOT-2 may be a limiting factor with regard to compliance, attention and ability to follow the standardized instructions. While the MABC-2 is simpler and takes less time to complete, the BOT-2 allows demonstration, provides visual supports (photos of required tasks), and structured instructions, which may make the BOT-2 a better option than the MABC-2 for children with ASD, if time permits to complete it during an evaluation (Case et al. 2019, Odeh et al. in preparation). The BOT-2 and MABC-2 both have timed components. The MABC-2 requires timing to completion of tasks while the BOT-2 requires counting number of repetitions completed in 15 s. Determining which tool to use clinically is challenging and should depend on the strengths and needs of the child as well as availability of the tool. The variability in performance on two different tools assessing similar components of motor skill is interesting though and should be investigated further.
Clinical evaluations must also focus on overall function and impact on daily living skills in addition to any motor skill assessments. Using parent-report tools or questionnaires is a way of getting additional information that does not require the attention and compliance of the child during the evaluation. It is also a valid and reliable way to get information on home and community functioning. Using the Vineland-3, the parents of the children with ASD in this study also reported decreased motor skills consistent with standardized tests. This shows that parents are capable of identifying motor skills that are known to impact daily living skills in children with ASD (Chatham et al. 2018, La Malfa et al. 2009).
Future research should also examine the cause of these motor deficits and their possible link with other diagnostic criteria for ASD. The growing body of evidence that individuals with ASD have an underlying motor component is becoming irrefutable. Because of the growing body of literature revealing motor impairments in children with ASD, physical therapists should be included as part of the interdisciplinary team to assist in the ongoing assessment and implementation of comprehensive therapeutic plans for children with ASD. Improved motor skills and confidence with balance and coordination tasks may facilitate increased participation in recreational activities and lead to a more active and healthier lifestyle.
Limitations
When considering the findings of this preliminary study, certain methodological limitations should be born in mind. First, the BOT-2 was always performed after the MABC-2 in an attempt to standardize the testing procedures. However, completion of the BOT-2 after the MABC-2 could contribute to fatigue, which may have contributed to the difference in findings between the two measures. In addition, children may have difficulty maintaining attention for a one- or two-hour session and may tire of the test. This is one of the reasons researchers allowed participants to complete the motor testing over two sessions. Reducing the variables of fatigue, attention and declining behavior was important. However, administering the tests over two sessions may also affect performance due to uncontrollable factors impacting the child’s mood, sleep, and willingness to participate day to day. Additionally, the motor assessments, when delivered in two separate sessions, were not necessarily performed at the same time of day. This was also accounted for by allowing the parent to schedule at a time convenient for them and at what they perceived to be an optimal time for their child. They were also provided the flexibility to reschedule, as needed. Although it is impossible to control for all variables, it was important to divide these sessions, as needed, to make sure each child was performing at his best level rather than invalidate scores due to poor test performance. Future studies should control for the presentation order to confirm that the order of administration does not alter the findings.
Second, this study only included a small sample of participants who would traditionally be described as higher functioning with average non-verbal IQ’s. Future studies should expand to investigate the motor proficiency of children with more significant cognitive and/or language impairment (Hirata et al. 2014). Because tasks that are timed and require specific directions may not be understood by children with ASD if their cognition or receptive language is impaired, it is imperative that the motor assessment allow for modifications, accommodations, or provide visual supports to get a more accurate performance of motor capabilities across the autism spectrum.
Conclusion
This preliminary study indicated that children with ASD between the ages of 5 and 12 have greater difficulty with global motor tasks when compared to their neurotypical peers across all motor assessment tools. While, overall, the children with ASD demonstrated deficits in tasks that targeted strength, speed, agility, coordination and both static and dynamic balance, it is critical to note that the range of skills in all domains demonstrated that not all children with ASD have difficulty with motor skills or daily living skills. This is a key argument for the continuation of individualized plans for intervention and the use of clinical evaluative tools as only one piece of evidence for clinical decision-making. Although manifestations of motor skill deficits specific to the ASD population are variable, physical therapists should be included in the ongoing assessment and implementation of comprehensive therapeutic plans for children with ASD to provide individually tailored care for each child’s motor profile.
Acknowledgements
Thank you to all of the children and families that participated in this study and to alumni Alyssa Wunderlich and Erik Johnson for their contributions in recruitment and data collection.
Funding Statement
This research was supported by the NIU College of Health & Human Sciences, The NIU Division of Research and Innovative Partnerships, and the NIU Center for the Interdisciplinary Study of Language and Literacy.
Conflict of interest
The authors declare no conflicts of interest.
References
- Ament, K., Mejia, A., Buhlman, R., Erklin, S., Caffo, B., Mostofsky, S. and Wodka, E.. 2015. Evidence for specificity of motor impairments in catching and balance in children with autism. Journal of Autism and Developmental Disorders, 45, 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni, G., Tasso, A., Muratori, F. and Cubelli, R.. 2016. The Vineland-II in preschool children with autism spectrum disorders: An item content category analysis. Journal of Autism and Developmental Disorders, 46, 42–52. [DOI] [PubMed] [Google Scholar]
- Brown, L., Sherbenou, R. J. and Johnsen, S. K.. 1997. Test of nonverbal intelligence, third edition examiner’s manual. Austin, TX: Pro-Ed. [Google Scholar]
- Bruininks, R. H. and Bruininks, B. D.. 2005. Bruininks-Oseretsky test of motor proficiency. 2nd ed. Minneapolis, Minnesota: Pearson Assessments. [Google Scholar]
- Buchner, A.Erdfelder, E.Faul, F. and Lang, A. G.. 2017. G*Power: Statistical power analyses for Windows and Mac (Version 3.1.9.2 for Windows): Heinrich-Heine-Universität Düsseldorf.
- Case, L., Schram, B. and Yun, J.. 2019. Motivating children with autism spectrum disorder in gross motor-skill assessments. Journal of Physical Education, Recreation & Dance, 90, 32–38. [Google Scholar]
- Chatham, C. H., Taylor, K. I., Charman, T., Liogier D'ardhuy, X., Eule, E., Fedele, A., Hardan, A. Y., Loth, E., Murtagh, L., del Valle Rubido, M., San Jose Caceres, A., Sevigny, J., Sikich, L., Snyder, L., Tillmann, J. E., Ventola, P. E., Walton-Bowen, K. L., Wang, P. P., Willgoss, T. and Bolognani, F.. 2018. Adaptive behavior in autism: Minimal clinically important differences on the Vineland-II. Autism Research, 11, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaine, M., Hollard, G. and Daunizeau, J.. 2014. Theory of mind: Did evolution fool us? PLOS One, 9, e87619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey, R. and Rapport, M. J. K.. 2012. Motor activity in children with autism: A review of current literature. Pediatric Physical Therapy, 24, 2–20. [DOI] [PubMed] [Google Scholar]
- Duvekot, J., Van Der Ende, J., Verhulst, F. C. and Greaves-Lord, K.. 2015. The Screening Accuracy of the Parent and Teacher-Reported Social Responsiveness Scale (SRS): Comparison with the 3Di and ADOS. Journal of Autism and Developmental Disorders, 45, 1658–1672. doi: 10.1007/s10803-014-2323-3. [DOI] [PubMed] [Google Scholar]
- Farmer, C., Adedipe, D., Bal, V. H., Chlebowski, C. and Thurm, A.. 2020. Concordance of the Vineland adaptive behavior scales, second and third editions. Journal of Intellectual Disability Research, 64, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F., Erdfelder, E., Lang, A.-G. and Buchner, A.. 2007. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Flanagan, J., Landa, R., Bhat, A. and Bauman, M.. 2012. Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy, 66, 577–585. [DOI] [PubMed] [Google Scholar]
- Foster, N. C., Bennett, S. J., Causer, J., Elliott, D., Bird, G. and Hayes, S. J.. 2020. Getting off to a shaky start: Specificity in planning and feedforward control during sensorimotor learning in autism spectrum disorder. Autism Research, 13, 423–435. [DOI] [PubMed] [Google Scholar]
- Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N. and Cauraugh, J. H.. 2010. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40, 1227–1240. [DOI] [PubMed] [Google Scholar]
- Ganglmayer, K., Schuwerk, T., Sodian, B. and Paulus, M.. 2019. Do children and adults with autism spectrum condition anticipate others’ actions as goal-directed? A predictive coding perspective. Journal of Autism and Developmental Disorders, 1–13. doi: 10.1007/s10803-019-03964-8 [DOI] [PubMed] [Google Scholar]
- Gladfelter, A., Johnson, E. and Odeh, C. E.. 2020. Parent perceptions of social behaviors associated with autism spectrum disorder are related to motor skills. Communication Disorders Quarterly, 41, 193–194. [Google Scholar]
- Gordon, R. G. and Watson, L. R.. 2015. Brief report: Gestures in children at risk for autism spectrum disorders. Journal of Autism and Developmental Disorders, 45, 2267–2273. [DOI] [PubMed] [Google Scholar]
- Griffiths, A., Toovey, R., Morgan, P. E. and Spittle, A. J.. 2018. Psychometric properties of gross motor assessment tools for children: A systematic review. BMJ Open, 8, e021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D., Charman, T., Pickles, A., Chandler, S., Loucas, T., Simonoff, E. and Baird, G.. 2009. Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 51, 311–316. [DOI] [PubMed] [Google Scholar]
- Guinchat, V., Chamak, B., Bonniau, B., Bodeau, N., Perisse, D., Cohen, D. and Danion, A.. 2012. Very early signs of autism reported by parents include many concerns not specific to autism criteria. Research in Autism Spectrum Disorders, 6, 589–601. [Google Scholar]
- Hadder-Algra, M. 2018. Early human motor development: From variation to the ability to vary and adapt. Neuroscience & Biobehavioral Reviews, 90, 411–427. [DOI] [PubMed] [Google Scholar]
- Hannant, P., Cassidy, S., Van de Weyer, R. and Mooncey, S.. 2018. Sensory and motor differences in autism spectrum conditions and developmental coordination disorder in children: A cross-syndrome study. Human Movement Science, 58, 108–118. [DOI] [PubMed] [Google Scholar]
- Henderson, S., Sugden, D., Barnett, A., Petermann, F., BöS, K. and Jascenoka, J.. 2015. Movement assessment battery for children: 2nd Edition (MABC-2). Bloomington, Minnesota: Pearson. [Google Scholar]
- Hirata, S.Nakai, A.Okuzumi, H.Kitajima, Y.Hosobuchi, T. and Kokubun, M.. 2015. Motor skills and social impairments in children with autism spectrum disorders: A pilot study using the Japanese version of the developmental coordination disorder questionnaire (DCDQ-J). SAGE Open, 5, 1–7. [Accessed Jan 2020] [Google Scholar]
- Hirata, S., Okuzumi, H., Kitajima, Y., Hosobuchi, T., Nakai, A. and Kokubun, M.. 2014. Relationship between motor skill and social impairment in children with autism spectrum disorders. International Journal of Developmental Disabilities, 60, 251–256. [Google Scholar]
- Holloway, J. M., Long, T. M. and Biasini, F.. 2018. Relationships between gross motor skills and social function in young boys with autism spectrum disorder. Pediatric Physical Therapy: The Official Publication of the Section on Pediatrics of the American Physical Therapy Association, 30, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, L., Hill, E. and Hamilton, A. F. D. C.. 2016. The relationship between social and motor cognition in primary school age-children. Frontiers in Psychology, 7, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos, A. D. and Givens, D. L.. 2018. Exercise for impaired balance. In: Duffield M.A, ed. Therapeutic exercise: Foundations and techniques. 7th ed. Philadelphia: F.A. Davis Company. Ch. 8. [Google Scholar]
- Kraft, K. P., Steel, K. A., Macmillan, F., Olson, R. and Merom, D.. 2015. Why few older adults participate in complex motor skills: A qualitative study of older adults’ perceptions of difficulty and challenge. BMC Public Health, 15, 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Malfa, G., Lassi, S., Bertelli, M., Albertini, G. and Dosen, A.. 2009. Emotional development and adaptive abilities in adults with intellectual disability. A correlation study between the scheme of appraisal of emotional development (SAED) and vineland adaptive behavior scale (VABS). Research in Developmental Disabilities, 30, 1406–1412. [DOI] [PubMed] [Google Scholar]
- Lane, H. and Brown, T.. 2015. Convergent validity of two motor skill test used to assess school-age children. Scandinavian Journal of Occupational Therapy, 22, 161–172. [DOI] [PubMed] [Google Scholar]
- Lang, R., Kern Koegel, L., Ashbaugh, K., Regester, A., Ence, W. and Smith, W.. 2010. Physical exercise and individuals with autism spectrum disorder: A systematic review. Research in Autism Spectrum Disorders, 4, 565–576. [Google Scholar]
- Leonard, H. C., Bedford, R., Pickles, A. and Hill, E. L.. 2015. Predicting the rate of language development from early motor skills in at-risk infants who develop autism spectrum disorder. Research in Autism Spectrum Disorders, 13-14, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C., DiLavore, P. C. and Gotham, K.. 2012. Autism diagnostic observation schedule. Torrance, CA: Western Psychological Services. [Google Scholar]
- May, T., McGinley, J., Murphy, A., Hinkley, T., Papadopoulos, N., Williams, K. J., McGillivray, J., Enticott, P. G., Leventer, R. J. and Rinehart, N. J.. 2016. A multidisciplinary perspective on motor impairment as an early behavioral marker in children with autism spectrum disorder. Australian Psychologist, 51, 296–303. [Google Scholar]
- Marquis, W. A. and Baker, B. L.. 2015. Sports participation of children with or without developmental delay: Prediction from child and family factors. Research in Developmental Disabilities, 37, 45–54. [DOI] [PubMed] [Google Scholar]
- McCoy, S. M., Jakicic, J. M. and Gibbs, B. B.. 2016. Comparison of obesity, physical activity, and sedentary behaviors between adolescents with autism spectrum disorders and without. Journal of Autism and Developmental Disorders, 46, 2317–2326. [DOI] [PubMed] [Google Scholar]
- Memari, A. H., Ghaheri, B., Ziaee, V., Kordi, R., Hafizi, S. and Moshayedi, P.. 2013. Physical activity in children and adolescents with autism assessed by triaxial accelerometry. Pediatric Obesity, 8, 150–158. [DOI] [PubMed] [Google Scholar]
- Minshew, N., Sung, K., Jones, B. and Furman, J.. 2004. Underdevelopment of the postural control system in autism. Neurology, 63, 2056–2061. [DOI] [PubMed] [Google Scholar]
- Ozonoff, S., Young, G. S., Goldring, S., Greiss-Hess, L., Herrera, A. M., Steele, J., Macari, S., Hepburn, S. and Rogers, S. J.. 2008. Gross motor development, movement abnormalities and early identification of autism. Journal of Autism and Developmental Disorders, 38, 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, C. Y. 2009. Age, social engagement, and physical activity in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 3, 22–31. [Google Scholar]
- Pan, C. Y. 2014. Motor proficiency and physical fitness in adolescent males with and without autism spectrum disorders. Autism, 18, 156–165. [DOI] [PubMed] [Google Scholar]
- Paquet, A., Olliac, B., Golse, B. and Vaivre-Douret, L.. 2019. Nature of motor impairments in autism spectrum disorder: A comparison with developmental coordination disorder. Journal of Clinical and Experimental Neuropsychology, 41, 1–14. [DOI] [PubMed] [Google Scholar]
- Schmitz, C., Martineau, J., Barthélémy, C. and Assaiante, C.. 2003. Motor control and children with autism: Deficit of anticipatory function? Neuroscience Letters, 348, 17–20. [DOI] [PubMed] [Google Scholar]
- Schopler, E., Van Bourgondien, M., Wellman, G. J. and Love, S. R.. 2010. Childhood autism rating scale. 2nd ed. Los Angeles: Western Psychological Services. [Google Scholar]
- Solish, A., Perry, A. and Minnes, P.. 2010. Participation of children with and without disabilities in social, recreational and leisure activities. Journal of Applied Research in Intellectual Disabilities, 23, 226–236. [Google Scholar]
- Sparrow, S. S., Cicchetti, D. V. and Saulnier, C. A.. 2016. Vineland adaptive behavior scales (Vineland-3). 3rd ed. San Antonio, TX: Pearson. [Google Scholar]
- Tükel, Ş., Björelius, H., Henningsson, G., McAllister, A. and Eliasson, A. C.. 2015. Motor functions and adaptive behaviour in children with childhood apraxia of speech. International Journal of Speech-Language Pathology, 17, 470–480. [DOI] [PubMed] [Google Scholar]
- Veness, C., Prior, M., Bavin, E., Eadie, P., Cini, E. and Reilly, S.. 2012. Early indicators of autism spectrum disorders at 12 and 24 months of age: A prospective, longitudinal comparative study. Autism, 16, 163–177. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Hallac, R. R., Conroy, K. C., White, S. P., Kane, A. A., Collinsworth, A. L., Sweeney, J. A. and Mosconi, M. W.. 2016. Postural orientation and equilibrium processes associated with increased postural sway in autism spectrum disorder (ASD). Journal of Neurodevelopmental Disorders, 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt, C. and Craig, C.. 2012. Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42, 1799–1809. [DOI] [PubMed] [Google Scholar]
- Yimyang, D. P., Albury, R. A. and Leppert, M. L.. 2017. Do parental concerns predict developmental and behavioral diagnoses in a developmental clinic? Clinical Pediatrics, 56, 263–267. [DOI] [PubMed] [Google Scholar]