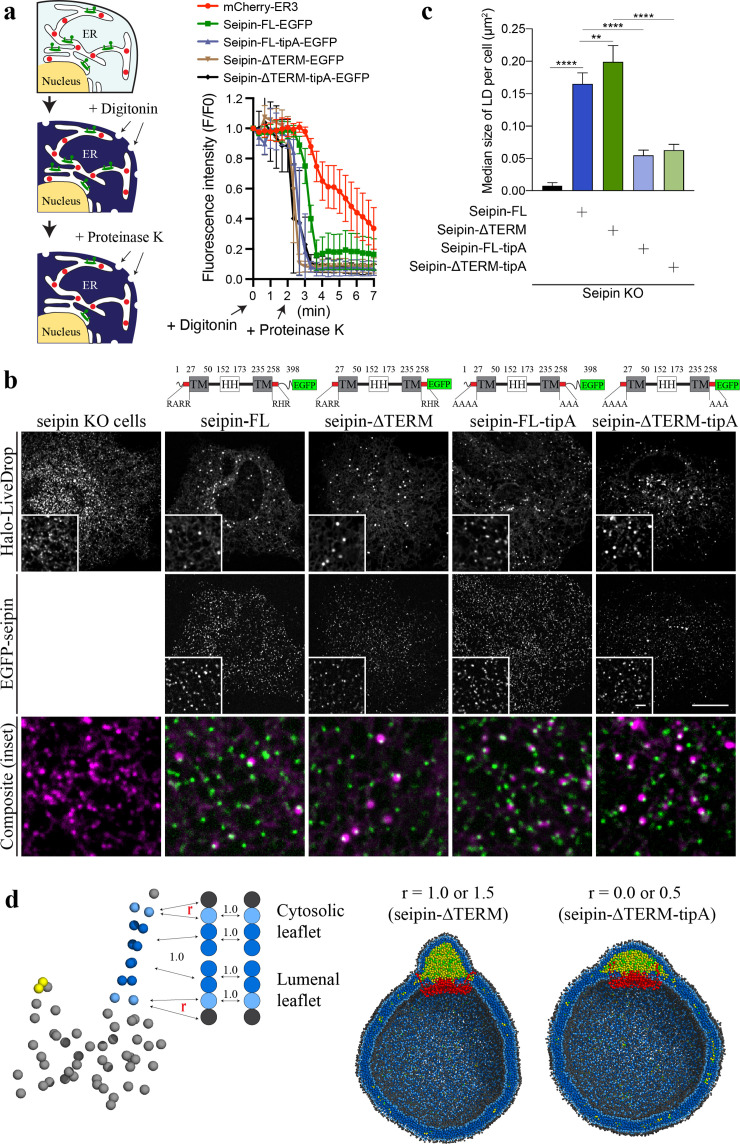
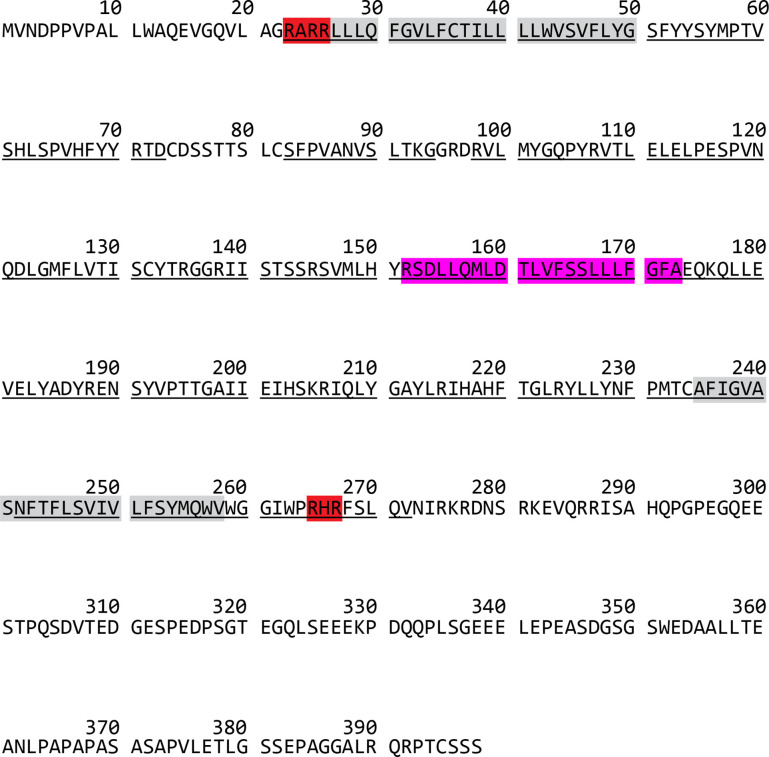
Figure 6. Nonconserved, cytosolic tails of human seipin are dispensable for the function, while the conserved, positively charged residues at the ends of seipin transmembrane (TM) segments are critical for lipid droplet (LD) maturation.
(a) Schematic representation of a fluorescence protease protection (FPP) assay (left). Low concentration of digitonin allows permeabilization of the plasma membrane without disrupting the endoplasmic reticulum (ER) membrane. Application of proteinase K selectively cleaves cytosolically exposed fluorescent protein (green) without affecting lumenally exposed fluorescent protein (red). Quantification of the fluorescence intensities of the whole time series of the FPP assay (right). (mean ± standard error of the mean [SEM]). (b) Confocal imaging of live seipin knockout (KO) SUM159 cells transiently transfected with various seipin constructs fused with EGFP and Halo-LiveDrop (stained with JF549). The cells were preincubated with 0.5 mM oleic acid for 1 hr prior to image acquisition. Scale bars, full-size, 15 μm; inserts, 2 μm. (c) Quantification of size of LDs per cell shown in (b) n = 4 cells. More than 300 LDs were analyzed in each sample. Median with interquartile range. ****p < 0.0001, **p < 0.01 were calculated by unpaired t-test. (d) CG molecular dynamics (MD) simulations with various attraction scaling factors (r). The spherical bilayer has a diameter of 40 nm and contains 6% mol TG. The elastic network model (ENM) model of human seipin with a spring constant of 0.2 kcal/mol/Å2 was used.