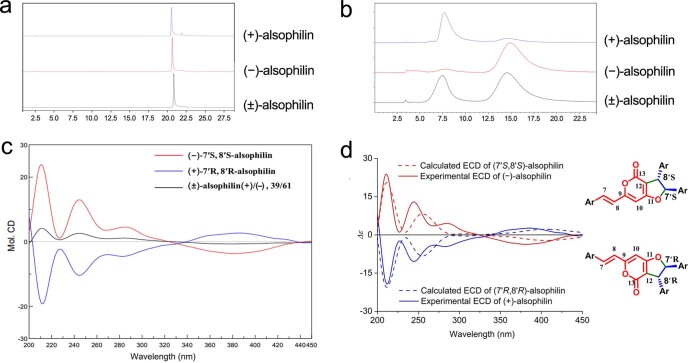
Extended Data Fig. 6. HPLC analysis and ECD calculation for the enantiomers (±)-alsophilin.
(a) HPLC analysis of (±)-alsophilin, (−)-alsophilin, and (+)-alsophilin on a C18 OSAKA SODA CAPCELL PAK column (150 × 4.6 mm I.D., 5 µm) using water (solvent A) and acetonitrile (solvent B) as gradient eluent (0-30 min, 10%-50% B; 30-35 min, 50%-100% B), flow rate 1 ml/min, at 382 nm. (b) HPLC analysis of (±)-alsophilin, (−)-alsophilin, and (+)-alsophilin on a chiral column Daicel Chiralpak IC column (250 × 4.6 mm I.D., 5 µm) using isopropyl and hexane as eluent (60:40) at a flow rate of 1 ml/min. (c) Circular dichroism (CD) spectra of (±)-alsophilin, (−)-alsophilin, and (+)-alsophilin in MeOH, measured using JASCO J-815 CD spectro polarimeters. (d) Comparison of the calculated ECD spectra for (7′S,8′S)-alsophilin and (7′R,8′R)-alsophilin with the experimental spectra of (−)-alsophilin and (+)-alsophilin in MeOH. Energies of the conformers of (+)-alsophilin at B3LYP/6-311g (d,p) in MeOH are shown in Supplementary Table 20.