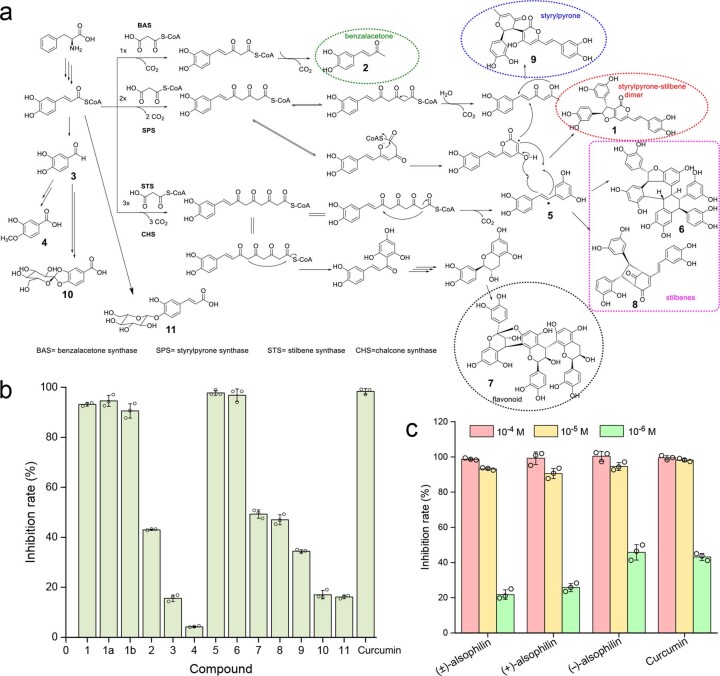
Extended Data Fig. 9. Chemical structures and antioxidant activities of 11 secondary metabolites isolated from A. spinulosa stems.
(a) Chemical structure and hypothetical biosynthetic pathway of one new compound (1) and ten known compounds (2-11). compound 1: (±)-alsophilin; compound 2: 3,4-dihydroxybenzalacetone; compound 3: protocatechnic aldehyde; compound 4: vanillic acid; compound 5: piceatannol; compound 6: cyperusphenol B; compound 7: cinnamtannin B-1; compound 8: jezonodione; compound 9: davallialactone; compound 10: cyathenosin A; compound 11: 4-O-β-D-glucopyranosyl-p-coumaric acid. (b) Antioxidant effects on MDA production of pure compounds at 10−5 M, using curcumin as the positive control. compound 1a: (−)-alsophilin; compound 1b: (+)-alsophilin. (c) Antioxidant effects on MDA production of (±)-alsophilin, (−)-alsophilin, and (+)-alsophilin at 10−4, 10−5, and 10−6 M, respectively, using curcumin as positive control. The data of inhibition rates in b and c are presented as means ±SD of three independent experiments.