Abstract
The gut contains the largest macrophage pool in the body, with populations of macrophages residing in the mucosa and muscularis propria of the gastrointestinal (GI) tract. Muscularis macrophages (MMs), which are located within the muscularis propria, interact with cells essential for GI function, such as interstitial cells of Cajal, enteric neurons, smooth muscle cells, enteric glia, and fibroblast-like cells, suggesting that these immune cells contribute to several aspects of GI function. This review focuses on the latest insights on the factors contributing to MM heterogeneity and the functional interaction of MMs with other cell types essential for GI function. This review integrates the latest findings on macrophages in other organs with increasing knowledge of MMs to better understand their role in a healthy and diseased gut. We describe the factors that contribute to (muscularis macrophage) MM heterogeneity, and the nature of MM interactions with cells regulating GI function. Finally, we also describe the increasing evidence suggesting a critical role of another immune cell type, the mast cell, in normal and diseased GI physiology.
Keywords: muscularis macrophages, enteric neurons, cell to cell communication, gastrointestinal functional disorders, mast cells
Abbreviations used in this paper: α-Syn, α-synuclein; CB, cannabinoid; CCR2, C-C Motif Chemokine Receptor 2; CD, cluster of differentiation; CGRP, calcitonin gene–related peptide; CNS, central nervous system; CSF1, colony-stimulating factor 1; Cx, connexin; CX3CR1, C-X3-C Motif Chemokine Receptor 1; EGC, enteric glial cell; EN, enteric neuron; GFAP, Glial Fibrillary Acidic Protein; GI, gastrointestinal; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; ICC, interstitial cells of Cajal; IFN-γ, interferon γ; IL, interleukin; LPS, lipopolysaccharide; MC, mast cell; MHCII, major histocompatibility complex II; MM, muscularis macrophage; POI, postoperative ileus; SP, substance P; TNF-α, tumor necrosis factor α; WT, wild-type
Summary.
This review describes the factors contributing to muscularis macrophage heterogeneity and the nature of muscularis macrophage’s interactions with cells that regulate gastrointestinal function. The emerging role of mast cells in gastrointestinal homeostasis and diseases also is described.
Early studies based on electron microscopy and immunohistochemistry identified “macrophage-like cells” in the muscularis propria of the gut.1, 2, 3, 4, 5 From their first identification, the progression of technology led to a more complex understanding of the role of muscularis macrophages (MMs) in gastrointestinal (GI) homeostasis and disease development and their heterogeneity. For years, the macrophage's phenotype relied on the classic vs alternative activation nomenclature based primarily on macrophage activation to in vitro signals.6,7 After treatment with lipopolysaccharide (LPS) and interferon-γ (IFN-γ), macrophages acquire a proinflammatory phenotype called “classically activated.”8 On the other side of the spectrum, the treatment of macrophages with interleukin (IL)4 or IL13 promotes an anti-inflammatory phenotype called “alternative activated.”9
Recently, this phenotypic dichotomy has been surpassed and replaced by a more complex network model that considers the cellular changes within a specific tissue and the numerous stimuli macrophages undergo, resulting in a continuum of phenotypes and more plasticity than previously recognized10,11
MMs express multiple markers as microglia, such as major histocompatibility complex II (MHCII), cluster of differentiation 11c (CD11c), cluster of differentiation 103 (CD103), and cluster of differentiation (CD11b+).11,12 As in other tissues, most MMs at steady-state conditions express a high level of C-X3-C Motif Chemokine Receptor 1 (CX3CR1) combined with colony-stimulating factor 1 (CSF1) and Fcγ receptor. The importance and role of CSF1 in the development and maintenance of MMs is highlighted by the near-complete absence of MMs in Csf1op/op mice, lacking the gene encoding for CSF1 from birth.13,14 MMs share a general anti-inflammatory phenotype at steady-state conditions compared with mucosal macrophages, which, in contrast, have an overall inflammatory phenotype.15,16 This may be a consequence of the location of MMs because the mucosa is constantly exposed to external stimuli. In addition, MMs share wound healing and tissue-protective genes such as Rental (encoding Fizz1), Mrc1, Cd163, and Il10.17,18 Contrary to the increasing understanding of the murine MM phenotype complexity, human MM information is limited and relies heavily on immunohistochemistry and morphology-based studies. Similar to the observations obtained in mice, transition of monocytes to a tissue-resident phenotype has been described in mucosal macrophages, where a population of monocytes sharing CD14high C-C Motif Chemokine Receptor 2 (CCR2+)CD11chigh markers is transitioning to a CD14lowCCR2-CD11chigh macrophage phenotype.19
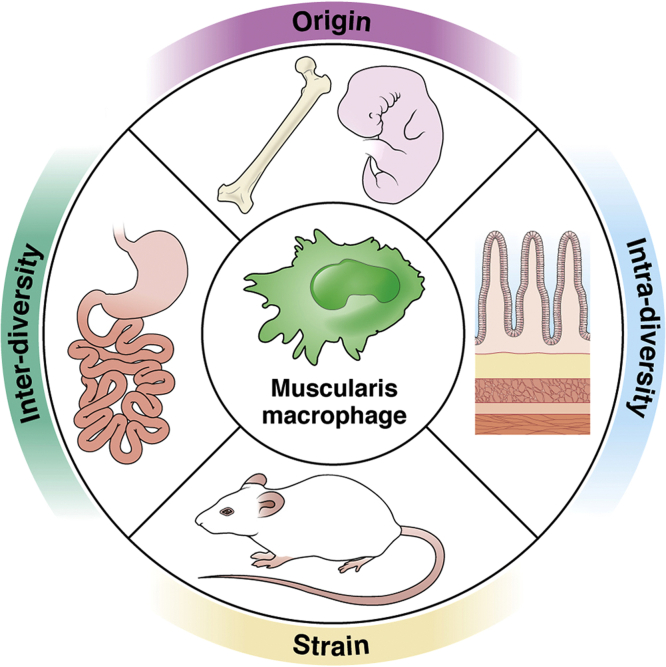
Further investigations are needed to provide information about the composition and distribution of human MMs to understand the degree of similarities with murine MMs. Overall, the heterogeneity of the MM phenotype (Figure 1) depends mostly on the origin and location in different regions of the gut (interdiversity) and between different regions across the muscularis propria (intradiversity).
Figure 1.
MM heterogeneity. Overview of the different factors contributing to MM diversity.
Origin
For a long time, it has been thought that resident macrophages were continuously replenished by circulating monocytes.20 In the past decades, investigators have challenged this concept, suggesting that macrophage heterogeneity within tissues depends on different cell origins. It is now clear that resident macrophages are established before birth in several organs and can maintain their number by cell division without monocyte recruitment.21,22 Recently, an elegant report23 identified a population of MMs in the small intestine that engrafted the GI tract during the embryonic state. This population of embryonic origin is known as long-lived MMs because it persists in the muscularis propria depending exclusively on cell proliferation. Besides this population, throughout life, the muscularis propria is populated primarily by monocyte-derived MMs that continuously replenish tissue-resident MMs. Monocyte- and embryonic-derived MMs represent the entire pool of tissue-resident MMs. In the same study, the investigators provided evidence that this population of embryonic origin clusters near enteric neurons (ENs) and regulates their number. Pieces of evidence suggest the possible contribution of monocyte-derived MMs to GI dysfunction. CCR2-dependent monocyte-derived macrophages play a role in resolving and restoring GI motility in postoperative ileus.24
In diabetic mice with gastroparesis, an increased number of MMs25 coincides with a higher level of MMs expressing proinflammatory markers,26 which can depend on increased recruitment of monocytes. A recent study27 identified a population of MMs closely associated with blood vessels in the GI muscularis called perivascular MMs. This population of MMs is regulated by the transcription factor Maf, which also controls the expression of several genes associated with the anti-inflammatory MMs. This population of MMs related to adipose tissue overlap with CD206 MMs. Interestingly, using a lineage tracing mouse model, the investigators clearly showed that this population is not dependent on circulating monocytes and primarly of embryonic origin. Conditional removal of the transcription factor c-MAF from MMs leads to CD206 macrophage loss.
Immune cell diversity has been described in 3 commonly used murine strains (C57BL/6NCr, 129/SvHsd, and BALB/cAnNCr).28 In this study, the investigators clearly showed a different macrophage phenotype in the spleen of the 3 different strains. In addition, transcriptomic and genetic analysis of macrophages from 5 different strains identified different levels of transcription factors leading to differences in the expression of genes29 driving macrophage phenotype. In addition, macrophages from BALB/cAnNCr and C57BL/6NCr respond differently to corneal transplantation.30 Almost all the information relative to MMs has been produced using C57BL/6NCr mice except for some studies on nonobese diabetic/ShiLtJ. Notably, as an indication of variability in MM phenotype owing to mouse strain, at steady-state conditions, nonobese diabetic mice do not express25 CD206, which otherwise is highly expressed in tissue-resident MMs in C57BL/6NCr mice.
Interdiversity and Intradiversity
As microglia in the central nervous system (CNS), MM phenotype depends on regional distribution across the smooth muscle layers and their interaction with other cell types populating the same environment (intradiversity). MMs are diverse and dynamic,31,32 and show a different morphology depending on their location. MMs can be divided into 3 distinct populations based on their distribution within the smooth muscle layers. MMs located in the myenteric plexus and serosal regions are multipolar, with many branches originating from the main body. MMs located within the muscular layers have a bipolar morphology following the muscle cell orientation. Further data are needed to understand if the morphologic differences between these diverse MM populations translate into functional changes.
A recent study23 associated a MM population with a specific area of the small intestine muscularis propria. De Schepper et al23 identified a MM population essential for EN maintenance, located within the myenteric plexus region where they interact with ENs. Although we have a clearer picture of MM distribution in different smooth muscle layers at steady-state conditions, we only have partial information about their distribution in states of altered homeostasis and disease. In aging,31 clusters of CD163-IR immune cells were visualized in proximity to sympathetic hyperinnervation of the jejunum of rats. In a mouse model of diabetic gastroparesis, an increase in MM number was described25 with the onset of diabetes, but no changes in the distribution of MMs has been reported. As briefly mentioned in the previous section, perivascular MMs27, associated with an anti-inflammatory and protective phenotype, are localized within the myenteric plexus region, where the blood vessels are located in the GI muscularis propria.
Another factor contributing to MM heterogeneity is the location of MMs within the different regions of the GI tract (interdiversity). We recently showed that MM distribution within the stomach and the small intestine presents some differences that require more attention in the future.32 MM distribution within the myenteric plexus and the smooth muscle layers were more homogenous in the stomach than in the small intestine. Gastric MMs are distributed evenly between the myenteric plexus and the smooth muscle layers, whereas small intestine MMs are distributed primarily in the myenteric plexus. Further studies are needed to understand if this critical difference in MM distribution also is responsible for functional changes. Phenotypically, at resting, gastric MMs do not express CD206 as MMs from the small intestine do. It also appeared that MMs in the different gut regions respond to external stimuli differently, suggesting a possible intrinsic phenotypic difference. For example, in diabetes, gastric MMs change their phenotype, leading to gastric dysfunction, whereas MMs in the small intestine appeared to be unchanged. More studies are needed to understand the differences between the populations of MMs residing in the different gut regions looking at the following: (1) phenotypic changes, (2) changes in response to inflammation/stimuli, and (3) origin.
Proximity Defines Functionality
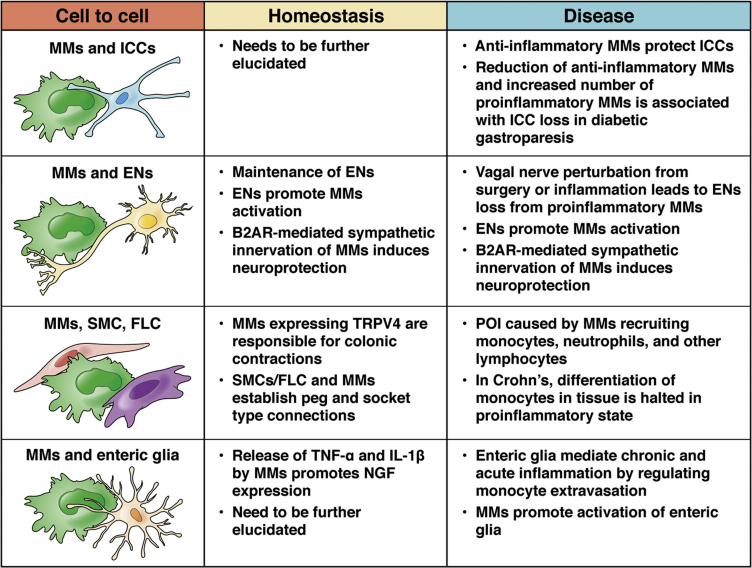
As previously described, one of the most critical factors contributing to MM heterogeneity is the tissue cue where MMs reside and interact with different cell types. MMs share the muscularis propria environment with ENs, interstitial cells of Cajal (ICC), glial cells, smooth muscle cells, and fibroblast-like cells, contributing to GI physiology (Figure 2). This type of interaction also has been described in the CNS, where microglia functionally interact with neurons and astrocytes, representing a multicellular system close to that observed in the enteric nervous system. Therefore, we will use this parallelism with microglia to describe the latest information about MMs' interaction with important cells for GI physiology and to underline areas that could be investigated further in the future
Figure 2.
Cell-to-cell interaction between MMs and cells required for GI contractility. MMs establish multiple functional interactions with cells important for GI function. B2AR, beta-2 adrenergic receptor ; FLC, Fibroblast like cell; NGF, nerve growth factor; SMC, smooth muscle cell; TRPV4, transient receptor potential vanilloid-type 4.
MMs/Enteric Glia
In the CNS, astrocytes and oligodendrocytes are macroglia, and both maintain the neuron's work environment by keeping neurotransmitters around synapses and providing support to axons.33,34 Enteric glial cells (EGCs) are found in the enteric nervous system surrounding the ENs35, 36, 37, 38, 39, 40 and share similar features with the brain macroglia,41 represented by astroglia and oligodendrocytes. As a consequence of their proximity to ENs, enteric glia contribute significantly to EN maintenance, survival, and function. Several studies in the CNS have described microglia–macroglia interaction. Activated microglia change astrocyte phenotype,41 providing the basis for developing new therapeutic strategies to target microglia-astrocytes in degenerative brain disease. Astrocyte–microglial interaction is required for synapse homeostasis, and the release of IL33 from astrocytes to microglia is required for maintaining neuron number and function.42 Different nerve injury types are associated with the activation of astrocytes and altered microglia phenotype.43 MMs lie in the proximity of enteric glial cells, suggesting a possible contribution of this interaction to GI physiology.
Dogiel44 described enteric glia in 1949, but only lately have been defined as peripheral glia thanks to the accurate morphologic analysis conducted by Gabella.45 Enteric glia modulates neuronal circuits by providing neurotransmitter precursors and generating neuroactive substances.46 Because of this interaction, enteric glia's ablation leads to EN death.47 Similar studies to those in the CNS have suggested that enteric glial cells can change their phenotype to respond to the environment. Enteric glial cells respond to different cytokines such as IL4 and tumor necrosis factor α (TNF-α).48 Notably, incubation of cultured LPS-activated glial cells leads to increased expression of Glial Fibrillary Acidic Protein (GFAP+), which is independent of proliferation. The inhibition of the nuclear factor-κB pathway in glial cells can effectively ameliorate colonic inflammation in mice and cultured human biopsy specimens.48 In addition, enteric glia become activated during acute inflammation in vitro and in vivo.48 The increased number of CD68 MMs during inflammation, partially owing to circulating monocyte extravasation, is reduced after conditional depletion of connexin (Cx)43, a gene that encodes for gap junctions, from enteric glial cells (EGCs).49 IL1β level, which is increased in inflammation, binds to its receptor on EGCs and regulates CSF1 expression by a Cx43-dependent mechanism.50
A similar mechanism involving EGC-MM crosstalk also was observed in postoperative ileus (POI), which is associated with an increased level of IL1β. Intraperitoneal injection of IL1β promoted the expression of proinflammatory genes, and mice deficient for IL1R1, a receptor for IL1β, are protected from POI. Interestingly, immunohistochemistry study showed that Interleukin 1 Receptor Type 1 (IL1R1) in POI co-labeled with EGC labeled with GFAP. EGCs stimulated with 10 ng/mL IL1β in-vitro for 24 hours expressed a high level of monocyte chemoattractant protein-1 (MCP-1), suggesting the possible involvement of these cells on circulating monocyte recruitment.51 Another study showed that EGCs, after inflammation, express CCL2, which promotes monocyte recruitment upon binding with its receptor CCR2.52
In colitis induced by Heligmosomoides polygyrus infection, transcriptome analysis of isolated MMs showed the enrichment of IFN-γ in the colon. These data are consistent with an enrichment of IFN-γ from EGCs from patients undergoing inflammation. Interestingly, IFN-γ drives a feedback effect on EGCs by eliciting chemokine IFN-γ–inducible protein 10 kilodalton (CXCL10) and guanylate-binding protein 10 expression through signal transducer and activator of transcription 1, leading to reduced proliferation of EGCs. CXCL10 and guanylate-binding protein 10 are involved in host defense and mediate immune responses with regard to antibacterial immunity and cancer, respectively.53 In addition to directly impacting EGCs, the IFN-γ–EGC–Cxcl10 signaling axis regulates tissue repair after helminth infection through MMs via CCL8, CCL7, CXCL2, and CCL2 activation.
α-synuclein (α-Syn) aggregates are found in the brain of patients with Alzheimer's disease. Most patients with this disease suffer from GI functional disorders, however, the mechanism is not understood. Application of α-Syn aggregates into the muscularis propria promotes the expansion of EGCs and overall tissue inflammation. Although it is not directly tested, it is possible that in this context, EGCs orchestrate the overall α-Syn–mediated inflammation by talking with MMs because multiple genes expressed after EGC expansion are associated with MMs.54
Proinflammatory MM products, such as IL1, IL4, and TNF-α, promote EGC activation such as reactive gliosis in the CNS. It also is evident that MM products can affect EGC phenotype during inflammation. Esposito et al48 showed that upon LPS treatment, EGCs acquire an activated phenotype that coordinates the inflammatory response in the enteric nervous system. Inhibiting the nuclear factor-κB pathway on EGCs ameliorated the overall inflammatory response in colitis and in vitro models. On the other hand, treatment of EGCs in vitro with LPS promoted the expression of genes associated with an anti-inflammatory response.
MMs/ENs
Intrinsic innervation
A microglia/neuron interaction has been described in the healthy and diseased brain. CX3CR1 is present in neurons' membrane, and its expression level is associated with stroke pathophysiology. The CX3CL1/CX3CR1 axis mediates microglial activation and plays a detrimental role in mice with ischemia.55 Because of this interaction, CΧ3CR1 null mice show an increased number of immature and dendritic spines as a result of microglia absence.56 Microglia modify neuronal membrane properties and functionality.57
The role of MM–EN functional interactions in the GI tract has been studied extensively. One of the oldest dogmas in the neurogastroenterology field on the stability of the neuronal circuits was challenged recently by Kulkarni et al.58 In this study, the investigators suggested that the number of ENs in adults results from a dynamic balance between EN death by apoptosis and the continuous production of ENs by neurogenesis. Interestingly, MMs, which are near ENs, phagocyte ENs that undergo apoptosis, as MHCII co-labeled with ENs by flow cytometry, and portions of apoptotic ENs are found within the MHCII+ nuclei.
In the colon and small intestine, MMs interact with ENs, releasing bone morphogenetic protein 2 (BMP2) upon release of CSF1 from ENs, a system regulated by the microbiome.13 Depletion of MMs results in poorly coordinated colonic contractions in an ex vivo model and abnormal colonic transit time in vivo. The addition of exogenous bone morphogenetic protein 2 to the colonic rings of MM-deficient mice decreases stretch-induced contractions. CSf1op/op mice, which do not have MMs from birth, have an abnormal myenteric plexus and more ENs than control mice.
Although the number of nitrergic ENs is increased in CSf1op/op mice, the number of cholinergic ENs is not altered, suggesting that MMs may regulate different subtypes of ENs.59 In the same animal model, we also showed that the absence of MMs from birth is associated with more ENs sharing cholinergic and nitrergic phenotypes, indicative of a more undifferentiated population of ENs. A reduced number of anti-inflammatory MMs in aged mice is linked to EN loss.60,61 In another report, the investigators tested the association of MMs with ENs during development using a double-transgenic zebrafish model. This study provided evidence of the intimate proximity between MMs and ENs starting at 3 days after fertilization. The number of total MMs that colonize the tissue increased, and more MMs associate with ENs. Furthermore, conditional depletion of Interferon Regulatory Factor 8 (irf8) from MMs reduced the total number of MMs during development, leading to faster intestinal transit.62
Extrinsic innervation
The amount of data describing the functional interaction between tissue macrophages and peripheral nerves are limited, mainly because the number of nerve-associated macrophages is minimal compared with the total number of tissue macrophages. For example, macrophages expressing CX3CR1 are closely associated with sympathetic nerve fibers of adipose tissue.63,64 A population of macrophages also has been described in the proximity of dermal sensory nerves in the skin.64 Precise extrinsic afferent (visceral sensory) and efferent (sympathetic and parasympathetic) innervation of the gut is fundamental for gut–brain crosstalk. Although the extrinsic nerves are not directly modulating gut motility, they can affect it by regulating other cell types within the enteric nervous system. Interactions between MMs and extrinsic innervation65 and the effect of sympathetic and catecholaminergic signaling in the immune cells' modulation have been studied extensively in the past. However, their possible involvement in modulating MM polarization phenotype in the gut has been described only recently.
In their study, Gabanyi et al15 suggest the regulation of MM activation by the beta-2 adrenergic receptor (β2AR). β2AR+ MMs reside near neuronal cell bodies or processes of the myenteric ganglia. Because of this interaction, MMs express higher levels of β2AR, a neuropeptide receptor, than lamina propria macrophages. Notably, adrenergic signaling through this receptor reduces ENs loss after infection.66 Acetylcholine represents the primary parasympathetic neurotransmitter released by preganglionic nerve fibers and the vagus nerve. Acetylcholine (ACh) has been studied for its anti-inflammatory effects in the periphery, where its stimulation was sufficient to suppress systemic inflammation in response to endotoxin.67 With this discovery, mice lacking α7 nicotinic acetylcholine (α7 nAChR) have increased systemic levels of TNF-α, IL1β, and Il6 in the skin.68 Cholinergic neuronal release of acetylcholine during vagal nerve stimulation promotes an anti-inflammatory macrophage phenotype via the α7 nAChR. It results in the amelioration of muscular inflammation.69,70 In addition, vagal manipulation promotes an increased number of gastric MHCII MMs, resulting in delayed gastric emptying.71 In the CNS, the type of communication microglia establishes with nerve fibers or the cell body is different. Recently, a beautiful study suggested that microglia monitor and protect neuronal function through specialized somatic purinergic junctions when communicating with cell bodies.72
Because of their location, MMs establish interaction with both nerve fibers and neuronal bodies across the smooth muscle layers; however, we still are far from understanding if, as described in the CNS, the type of communication between nerve fibers and cell bodies is anatomically and functionally different. The nature of communication between MMs and ENs has been the most described in the field, but we still are far from deciphering MM–EN communication in homeostasis and diseases. Because many functional diseases have been associated with EN number reduction, understanding the contribution of MMs to this phenomenon represents a fundamental step to build future and promising therapeutic strategies to regulate and maintain ENs in functional diseases characterized by EN loss.
MMs/ICC
For many years, ICC were characterized only by nonspecific histologic stains, and, later, more reliably, by electron microscopy. The ultrastructural features and the ICC anatomic distributions suggested their critical physiological roles: (1) they are pacemaker cells and propagate slow waves,73,74 (2) they mediate both inhibitory and excitatory neurotransmission,75, 76, 77 and (3) they work as mechanosensors. A similar cell type is absent in the CNS, making this parallelism impossible. ICC role and function are similar to cells in the heart that represent the heart's pacemaker cells as the ICC for the gut. In a recent publication, Hulsmans et al78 described the functional interaction between macrophages and pacemaker cells in the heart. The study described an abundance of macrophages closely associated with the heart's AV nodes in homeostasis. Macrophages couple electrically with pacemaker cells through Cx43-containing gap junctions. The absence of this interaction (Cx43 knockout mice) results in impaired AV node conductance, suggesting the macrophages' critical role in facilitating the heart's electrical conduction. In the enteric nervous system, limited data describe MM–ICC interactions in homeostatic conditions. Electron microscopy and immunofluorescence analysis showed that MM populations are closely associated with ICC, suggesting a potential functional role for this type of interaction.1,2 In CSf1op/op mice that do not have macrophages from birth, ICC appeared to have a normal distribution,14 and the level of expression of Kit, a specific ICC marker, did not differ in comparison with controls. However, gastric emptying, which partly depends on ICC function, is faster than in control mice. The information relative to MM–ICC functional interactions in GI disease is more extensive. Blockade of IL17A signal reduced ICC loss in sepsis because of the reduction of proinflammatory MMs.79 In an inflammation model, numerous MMs were observed near ICC compared with controls at the ultrastructural level.80 In the same model with the progression of inflammation, macrophages presented large phagosomes and lysosomes in the proximity of injured ICC, indicating an ongoing phagocytic activity. During development, ICC release CSF1 in the small intestine, suggesting their possible contribution to MM migration and survival during this period.81 The bidirectional communication between ICC and MMs still needs to be elucidated. IL6 released by MMs during GI surgery promotes up-regulation of microRNA-19a responsible for ICC depletion.82 An increase of proinflammatory cytokines produced by MMs is associated with decreased c-kit–positive ICC in the dilated colon of Hirschsprung disease, associated with enterocolitis.83 Almost all of the information acquired in recent years describing a potential functional interaction between MMs and ICC has been produced in the context of diabetic gastroparesis, a functional disease affecting the stomach.
Conditioned media from proinflammatory activated macrophages reduces kit-positive ICC in vitro.84 The ineffectiveness of the same conditional media in the presence of a TNF-α neutralizing antibody implies this cytokine's implication in regulating kit expression.84 MM phenotype changes are associated with ICC loss and delayed gastric emptying in animal models and human beings.85,86 Patients with diabetic gastroparesis have fewer CD206+ MMs, a marker of anti-inflammatory macrophages. This reduction correlates with ICC loss,87,88 suggesting a protective role of anti-inflammatory MMs for ICC that is absent in diabetic gastroparesis. Anti-inflammatory MMs also secrete IL10 to induce heme oxygenase 1 expression, an enzyme that has a protective effect on ICC.87 Mice with delayed gastric emptying given exogenous IL10 return to regular gastric emptying with higher levels of heme oxygenase 1 and better-connected, more organized, and evenly distributed ICC networks.88 MM polarization to an anti-inflammatory phenotype may be a viable treatment for diabetic gastroparesis. Recently, we showed that the development of diabetes was associated with an increased level of proinflammatory MMs and reduced anti-inflammatory MMs. Notably, delayed gastric emptying and loss of ICC are related to lower protection exerted by the anti-inflammatory component and the increased level of expression of IL6 and inducible nitric oxide synthase (iNOS). A small number of data suggest the importance of MM–ICC functional interactions with other GI diseases. Chron's disease is associated with ICC injury and changes to MM morphology.89 In achalasia, ICC closely related to immune cells are preserved.90 Cytokines released by MMs have been considered responsible for ICC network disruption in the endothelin-B–receptor null rat, representing a model for long-segment Hirschsprung's disease.91
MMs/Smooth Muscle Cells and Fibroblast-Like Cells
Macrophages interact with smooth muscle cells in various organs. Conditioned medium from the macrophage cell line promotes differentiation of human adipose tissue–derived mesenchymal stem cells to smooth muscle cells.92 The interaction of macrophages with smooth muscle cells is responsible for extracellular matrix deposition during plaque progression. In addition, macrophages regulate vessel tone and diameters of blood pressure while interacting with vascular smooth muscle cells. However, the information of a potential interaction between MMs and smooth muscle cells in the gastrointestinal tract is, at best, limited. MMs expressed the transient receptor potential vanilloid 4 channel, and MMs' stimulation via transient receptor potential vanilloid 4 promotes prostaglandin E2 release, leading to colon contraction.93
LPS treatment, which induces MM polarization to a proinflammatory phenotype, reduces smooth muscle contractility.94,95 Treatment with a monocyte chemoattractant protein-1 reduces monocyte infiltration and leads to intestinal smooth muscle dysfunction.96 Notably, IL1β inhibits intestinal smooth muscle proliferation.97 Another type of interstitial cell in the gut that differs from ICC has been identified using antibodies against platelet-derived growth factor receptor-α.98,99 These cells are located between smooth muscle cells and intramural nerve fibers and play an essential role in neurotransmission.100 In addition to the expression of platelet-derived growth factor receptor-α, these cells differ from ICC at the ultrastructural level. These cells show a well-developed rough endoplasmic reticulum but do not have caveolae.101 In a recent publication,31 electron microscopy and immunohistochemistry showed that MMs establish cell-to-cell contact with fibroblast-like cells. The nature and the functional translation of these types of interaction need to be addressed in the future.
Interaction of Mast Cells With Enteric Neurons and Other Cells Required for Gastrointestinal Motility
As described earlier, the GI tract contains the largest pool of macrophages in the body. In addition, different types of immune cells are essential for developing normal intestinal morphology and function. Among that, mast cells (MCs) are certainly one of the most significant. MCs are principally localized in tissues that represent a barrier such as skin and respiratory and intestinal mucosa. At the same time, in the GI tract, their number is small, principally located in the mucosa (2%–3%) and the submucosa (approximately 1%) layers.102 However, MCs also can be recruited (mastocytosis) in response to specific stimuli.103 In addition, MCs contribute to intestinal functions (host defense, vascular permeability, peristalsis, and so forth).104,105 Thus, changes in their number or activity may lead to several pathologic states such as inflammatory bowel disease (IBD) or can impact gut functions, modifying sensory nociception, intestinal permeability, or altering motility and secretion.106
MCs are incredibly plastic and exert their functions almost exclusively by releasing mediators after activation. As reported by Buhner and Schemann,107 mediators released by MCs can be subdivided into 2 classes: preformed molecules, such as histamine and proteases that are stored as granules and therefore can be released within seconds; and mediators newly synthesized, produced after MC activation, such as lipid mediators and cytokines. Early anatomic studies described the nonrandom spatial association and membrane-to-membrane contacts between MCs and nerves in the human gut.108 Stead et al109,110 reported that an estimated 70% of MCs are in direct contact with nerves, and another 20% are within 2 μm. This anatomic association has functional relevance because communication between nerves and MCs is crucial for maintaining mucosal homeostasis and ensuring an appropriate response to injury, thanks to the expression of neurotransmitter and neuropeptide receptors by MCs and several receptors expressed by neurons.111 In guinea pig and human small intestine, the communication and interaction between MCs and peripheral nerves were meticulously described by Wang et al.112 As reported by the investigators, the relationship between spinal afferents and enteric MCs and their activation can be resumed at 5 pivotal points: (1) MCs expressed receptors for the neurotransmitters substance P (SP) and calcitonin gene–related peptide (CGRP); (2) after exposure to SP and CGRP, MCs released protease II and histamine; (3) histamine and protease II act in a diffuse paracrine manner to influence the behavior of ENs and spinal afferent terminals; (4) sensitization of the afferent terminals amplify MC release; and (5) MC-stabilizing drugs such as cromolyn and doxantrazole or neural blockade with (tetrodotoxin) TTX, suppressed the release of MC protease II and histamine. Furthermore, Buhner and Schemann107 recently showed that the mediators involved in nerve–MC interactions were CGRP and (vasoactive intestinal polypeptide) VIP rather than SP. Interestingly, Buhner and Schemann,107 divided the MC–nerve axis in the human gut, into MCs to nerve and nervetoMC signaling, underlining how activation of MCs causes mostly nerve sensitization, while nerves can activate or inhibit MC mediator release.
The reciprocal relationship between MCs and glial cells was first studied by Shalit et al113 in MCs co-cultured with confluent glial cell monolayers. In this experimental model, in the presence of IL3 (an important cytokine for the regulation of MC growth and differentiation),114 glial cells do not interfere with MC viability, function, and biochemical properties and these cells maintain their functional activity as shown with the release of histamine. In a model of stress owing to neonatal maternal separation and early weaning, linked to IBS,115 MCs were increased at the level of the myenteric plexus and produced more histamine than controls and were closely associated with enteric glia. The involvement of histamine in the glia–MC communication also was confirmed in an in vitro study on human EGCs obtained from surgical patients. The contribution of the histaminergic pathway in the pathogenesis of IBS is further pointed out by the observation that in the small intestine of IBS patients, the histamine N-methyltransferase is altered, producing an increased amount of histamine owing to lack of is degradation. MCs can influence and affect the numbers and the function of ICC. Reduced ICC are associated with more MCs in several pathologic states such as diabetic gastroparesis,116 Crohn's disease,89 and ulcerative colitis (UC)117
Mast Cells and Functional Diseases
There is strong evidence supporting nerve–MC interaction in diseases such as IBD and irritable bowel syndrome (IBS). In colon samples from dextran sulfate sodium (DSS)-treated rats, 5-hydroxytryptamine–containing MCs were close to CGRP-immunoreactive fibers in inflamed serosa, suggesting a functional association in the diseased state.118 Several studies have shown increased MC density in both IBD and IBS patients, and these cells could play a role in developing strictures and fistulas, irreversible complications of IBD.119 Studies on IBS colonic mucosa showed increased GFAP expression, indicating an enteric glial activation that was correlated negatively with MC density, suggesting altered EGC-MC interactions.120 Inflammation, even if mild, leads to persistent GI nerve changes, and these inflammation-induced changes persist after recovery. These changes could affect structural morphology (nerve bundles, hypertrophy, and/or hyperplasia) and neurotransmitter expression or receptor, such as a decreased release of acetylcholine and increased secretion of 5-hydroxytryptamine. Increased SP and neurokinin 1 (NK-1) receptors were reported in IBD patients and blocking of NK-1 receptors resulted in reductions of experimental colitis. Treatment with VIP after the onset of 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis in mice reduces colitis's clinical and histopathologic severity.121 In TNBS-induced colitis in rats, no differences in colonic damage, weight, and adhesion scores between wild-type (WT) and MC-deficient rats were observed.122 In the same model, nedocromil sodium, a MC stabilizer, reduced inflammation and fibrosis, possibly by decreasing MC numbers and activation and consequent collagen production. Compound 48/80, a MC activator, slightly enhanced the severity of the disease. In vitro, rat peritoneal MC increased fibroblast proliferation, collagen synthesis, and contractile activity, all hallmarks of fibrosis. These properties have been attributed to mediators such as tryptase, histamine, and TNF-α.123 On DSS-induced colitis and acute otitis media (AOM)/DSS-induced colorectal cancer, peptide F991, a functional inhibitor of free Ig light chain, significantly attenuated colitis progression and carcinogenesis, reduced the infiltration of inflammatory cells, and blocked inflammasome activation by inhibiting the cleavage of caspase-1 and the activation of IL1β and IL18.124 The severity of IBD triggered by exposure to piroxicam was similar in Il10-/- mice, in dystrophin-utrophin double knockout (DKO) mice with no MCs owing to the KitW-sh/KitW-sh mutation, and in DKO mice reconstituted with WT or Il10-/- bone marrow–derived MCs. However, DKO mice had significantly increased TNF-α and IFN-γ release, showing that MCs play a role in down-regulating proinflammatory cytokines within the inflamed colon.125 Thus, differences in results from different experimental models could be partly owing to differences between species (eg, rat vs mice), both in terms of localization and number of MCs.
MCs and POI
POI is a relatively common postoperative disorder. Although most patients recover in 2–3 days without medical intervention, in some cases, GI motility disorders last for a long time and prolong hospitalization, increasing medical costs. Surgical trauma and gut manipulation trigger an inflammatory response in the muscularis externa involving inflammatory cells, reactive enteric glia, neurons, smooth muscle cells, ICCs, epithelial cells, and the microbiome in the lumen of the gut.126 The recruitment of inflammatory cells is mediated by cellular adhesion molecules, including selectins, integrins, and adhesion molecules. Surgical manipulation causes a significant up-regulation of intercellular adhesion molecule-1 (ICAM-1) and P-selectin, and massive extravasation of leukocytes; the administration of adhesion molecule antibodies prevented the recruitment of monocytes and neutrophils, resulting in a significant reduction of all 3 populations of infiltrating leukocytes and prevented muscle dysfunction127 As reported earlier, complex communication pathways exist among neurons, glia, immune cells, and other cells, which may contribute to the pathogenesis of POI. Vagus nerve stimulation prevents POI in preclinical models by reducing nicotinic-receptor activation, and 5-Hydroxytryptamine Receptor 4 (5HT4R) agonists reduce the activation of MMs. Cholinergic neurons express cannabinoid (CB) receptors, and their activation inhibits the release of acetylcholine with subsequent slowing of intestinal motility and a delay in gastric emptying. Endogenous ligands of CB1 receptors attenuate inflammation by decreasing proinflammatory cytokine release and inhibiting MC degranulation. However, decreased GI transit and marked intestinal and systematic inflammatory responses were observed in both WT and CB1-/- mice after surgery. However, CB1-/- mice showed higher plasma levels of IL6, cytokine-induced neutrophil chemoattractant-1 (KC/CINC1)expression in mucosal epithelium and submucosa compared with WT mice.128 The contribution of MCs to POI was highlighted by experimental and clinical studies. Mechanical stretch results in the local release of sensory neurotransmitters, especially SP and CGRP, which can directly activate mast cells. Intestinal manipulation also elicited a significant increase in MC degranulation and gastroparesis, prevented by MC stabilizing agents.129 The involvement of MCs is shown further using the MC secretagogue C48/80. Moreover, intestinal manipulation in MC-deficient Kit/Kitv mice did not elicit significant leukocyte recruitment. Still, the reconstitution of the MC population in these animals restored the manipulation-induced inflammatory response to WT levels.130 Mouse models with abnormal Kit signaling also lack ICC, resulting in aberrant GI motility even before surgery. In a mouse model with selective depletion of MCs and typical ICC network, Cpa3Cre/+ mice, intestinal manipulation induced inflammation and delayed gut motility as in WT mice, which strongly argues against MCs as a crucial player in the development of POI, at least in mice.131 These investigators underlined that several issues such as nonspecific effects of MC degranulation or stabilization agents used in other studies may have led to misinterpretation of experimental data and incorrect conclusions. The et al131 reported that tryptase release in peritoneal lavage during abdominal surgery increased from 5.2 (basal level) to 23.1 in early samples and 51.7 mg/L at the end of surgery. In contrast, neither laparoscopic nor transvaginal intraperitoneal surgery elicited a significant MC response during 2 minimally invasive surgical procedures. Moreover, at the end of the surgical procedure, IL6 and IL8 increased while, in laparoscopy, only intraperitoneal IL8 was increased, but not as profoundly as in the laparotomy group. These findings in human beings support the hypothesis that the degree of intestinal handling, MC degranulation, and subsequent inflammation determines POI.
MCs and Achalasia
Achalasia is a motility disorder characterized by the absence of coordinated peristalsis and incomplete relaxation of the lower esophageal sphincter of unknown etiology. Liu et al132 determined the number of MCs, ICC, neuronal nitric oxides synthase (nNOS)-positive, and S-100–positive cells in lower esophageal sphincter muscle biopsy specimens from 116 patients with achalasia, observing an increased MC infiltration, significantly associated with decreased ICC, nNOS-positive, and S-100–positive cells.
Nelson et al133 did not observe differences in the lower esophageal sphincter MC density between patients and controls. However, almost all patients showed profound MC degranulation involving perimysium and myenteric plexus nerves. Moreover, patients with different manometric phenotypes showed different expression profiles: achalasia type II patients showed an up-regulation of genes that might increase cytosolic calcium and smooth muscle contraction. Both achalasia type I and type III (only 1) patients had up-regulated genes to replenish calcium stores in the sarcoplasmic reticulum. The aganglionic segment of the colon of Hirschsprung's disease patients showed an increased number of MCs, correlated with the number and perimeter of nerve fibers, suggesting a possible role of these cells in Hirschsprung's disease pathogenesis.134
Conclusion and Future Directions
MMs are specialized phagocytic cells that fulfill an important role in regulating GI homeostasis and disease. They contain different subpopulations whose phenotype depends on their GI tract location and origin. These unique MMs, compared with mucosal Ms, share an anti-inflammatory phenotype. It is evident now that MMs have a dual origin. The MM pool is maintained by both monocyte-derived and embryonic-derived MMs. Although there is evidence suggesting the involvement of embryonic-derived MMs in regulating ENs, no information supports the possible contribution of circulating monocytes to tissue homeostasis. Depending on their location, MMs can interact functionally with cells required for GI physiology. Further studies are needed to elucidate the underlying mechanisms regulating this type of interaction.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by an American Neurogastroenterology and Motility Society Young Investigator Award, American Gastroenterological Association Rome Foundation grant 36, and National Institute of Diabetes and Digestive and Kidney Diseases grants DK057061, DK68055, DK127992, and P30DK084567.
References
- 1.Rumessen J.J., Thuneberg L., Mikkelsen H.B. Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat. Rec. 1982;203(1):129–146. doi: 10.1002/ar.1092030112. [DOI] [PubMed] [Google Scholar]
- 2.Mikkelsen H.B., Thuneberg L., Rumessen J.J., Thorball N. Macrophage-like cells in the muscularis externa of mouse small intestine. Anat. Rec. 1985;213(1):77–86. doi: 10.1002/ar.1092130111. [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsen H.B. Macrophages in the external muscle layers of mammalian intestines. Histol. Histopathol. 1995;10:719–736. [PubMed] [Google Scholar]
- 4.Mikkelsen H.B., Thuneberg L., Wittrup I.H. Selective double staining of interstitial cells of Cajal and macrophage-like cells in small intestine by an improved supravital methylene blue technique combined with FITC-dextran uptake. Anat. Embryol. 1988;178:191–195. doi: 10.1007/BF00318222. [DOI] [PubMed] [Google Scholar]
- 5.Faussone-Pellegrini M.S., Pantalone D., Cortesini C. Smooth muscle cells, interstitial cells of Cajal and myenteric plexus interrelationships in the human colon. Acta Anat (Basel) 1990;139(1):31–44. doi: 10.1159/000146975. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 8.Yu S.F., Koerner T.J., Adams D.O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J. Leukoc. Biol. 1990;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]
- 9.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon S., Plüddemann A., Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol. Rev. 2014;262(1):36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda T., Sankowski R., Staszewski O., Böttcher C., Amann L., Sagar, Scheiwe C., Nessler S., Kunz P., van Loo G., Coenen V.A., Reinacher P.C., Michel A., Sure U., Gold R., Grün D., Priller J., Stadelmann C., Prinz M. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 12.Koscsó B., Gowda K., Schell T.D., Bogunovic M. Purification of dendritic cell and macrophage subsets from the normal mouse small intestine. J Immunol. Methods. 2015;421:1–13. doi: 10.1016/j.jim.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Muller P.A., Koscsó B., Rajani G.M., Stevanovic K., Berres M.L., Hashimoto D., Mortha A., Leboeuf M., Li X.M., Mucida D., Stanley E.R., Dahan S., Margolis K.G., Gershon M.D., Merad M., Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cipriani G., Gibbons S.J., Verhulst P.J., Choi K.M., Eisenman S.T., Hein S.S., Ordog T., Linden D.R., Szurszewski J.H., Farrugia G. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cell. Mol. Gastroenterol. Hepatol. 2016;2(1):40–47. doi: 10.1016/j.jcmgh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabanyi I., Muller P.A., Feighery L., Oliveira T.Y., Costa-Pinto F.A., Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164(3):378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mowat A.M., Bain C.C. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 2011;3(6):550–564. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehner S., Engel D.R. Resident macrophages in the healthy and inflamed intestinal muscularis externa. Pflugers Arch. – Eur. J. Physiol. 2017;469:541–552. doi: 10.1007/s00424-017-1948-4. [DOI] [PubMed] [Google Scholar]
- 18.Bujko A., Atlasy N., Landsverk O.J.B., Richter L., Yaqub S., Horneland R., Øyen O., Aandahl E.M., Aabakken L., Stunnenberg H.G., Bækkevold E.S., Jahnsen F.L. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J. Exp. Med. 2018;215(2):441–458. doi: 10.1084/jem.20170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardo D., Marin A.C., Fernández-Tomé S., et al. Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(-)CCR2(-)CX3CR1(-) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018;11:1114–1126. doi: 10.1038/s41385-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 20.van Furth R., Cohn Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacke F., Zimmermann H.W. Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Schepper S., Verheijden S., Aguilera-Lizarraga J., Viola M.F., Boesmans W., Stakenborg N., Voytyuk I., Schmidt I., Boeckx B., Dierckx de Casterlé I., Baekelandt V., Gonzalez Dominguez E., Mack M., Depoortere I., De Strooper B., Sprangers B., Himmelreich U., Soenen S., Guilliams M., Vanden Berghe P., Jones E., Lambrechts D., Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2019;176(3):676. doi: 10.1016/j.cell.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Farro G., Stakenborg M., Gomez-Pinilla P.J., Labeeuw E., Goverse G., Di Giovangiulio M., Stakenborg N., Meroni E., D'Errico F., Elkrim Y., Laoui D., Lisowski Z.M., Sauter K.A., Hume D.A., Van Ginderachter J.A., Boeckxstaens G.E., Matteoli G. CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut. 2017;66(12):2098–2109. doi: 10.1136/gutjnl-2016-313144. [DOI] [PubMed] [Google Scholar]
- 25.Choi K.M., Kashyap P.C., Dutta N., Stoltz G.J., Ordog T., Shea Donohue T., Bauer A.J., Linden D.R., Szurszewski J.H., Gibbons S.J., Farrugia G. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterol. 2010;138(7):2399–2409. doi: 10.1053/j.gastro.2010.02.014. 2409.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipriani G., Gibbons S.J., Miller K.E., Yang D.S., Terhaar M.L., Eisenman S.T., Ördög T., Linden D.R., Gajdos G.B., Szurszewski J.H., Farrugia G. Change in populations of macrophages promotes development of delayed gastric emptying in Mice. Gastroenterol. 2018;154(8):2122–2136. doi: 10.1053/j.gastro.2018.02.027. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moura Silva H., Kitoko J.Z., Queiroz C.P., Kroehling L., Matheis F., Yang K.L., Reis B.S., Ren-Fielding C., Littman D.R., Bozza M.T., Mucida D., Lafaille J.J. c-MAF-dependent perivascular macrophages regulate diet-induced metabolic syndrome. Sci Immunol. 2021 Oct;6(64):eabg7506. doi: 10.1126/sciimmunol.abg7506. [DOI] [PubMed] [Google Scholar]
- 28.Hensel J.A., Khattar V., Ashton R., Ponnazhagan S. Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab. Invest. 2019;99(1):93–106. doi: 10.1038/s41374-018-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Link V.M., Duttke S.H., Chun H.B., Holtman I.R., Westin E., Hoeksema M.A., Abe Y., Skola D., Romanoski C.E., Tao J., Fonseca G.J., Troutman T.D., Spann N.J., Strid T., Sakai M., Yu M., Hu R., Fang R., Metzler D., Ren B., Glass C.K. Analysis of genetically diverse macrophages reveals local and domain-wide mechanisms that control transcription factor binding and function. Cell. 2018;173(7):1796–1809. doi: 10.1016/j.cell.2018.04.018. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleul T., Zhuang X., Hildebrand A., Lange C., Böhringer D., Schlunck G., Reinhard T., Lapp T. Different innate immune responses in BALB/c and C57BL/6 strains following corneal transplantation. J. Innate Immun. 2021;13(1):49–59. doi: 10.1159/000509716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips R.J., Billingsley C.N., Powley T.L. Macrophages are unsuccessful in clearing aggregated alpha-synuclein from the gastrointestinal tract of healthy aged Fischer 344 rats. Anat Rec (Hoboken) 2013;296(4):654–669. doi: 10.1002/ar.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji S., Traini C., Mischopoulou M., Gibbons S.J., Ligresti G., Faussone-Pellegrini M.S., Sha L., Farrugia G., Vannucchi M.G., Cipriani G. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRα-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol. Motil. 2021;33(3) doi: 10.1111/nmo.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen N.J., Eroglu C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96(3):697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stieg P.E., Kimelberg H.K., Mazurkiewicz J.E., Banker G.A. Distribution of glial fibrillary acidic protein and fibronectin in primary astroglial cultures from rat brain. Brain Res. 1980;199(2):493–500. doi: 10.1016/0006-8993(80)90709-x. [DOI] [PubMed] [Google Scholar]
- 35.Bassotti G., Villanacci V., Antonelli E., Morelli A., Salerni B. Enteric glial cells: new players in gastrointestinal motility? Lab. Invest. 2007;87(7):628–632. doi: 10.1038/labinvest.3700564. [DOI] [PubMed] [Google Scholar]
- 36.Seguella L., Gulbransen B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021 March 17 doi: 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown I.A., McClain J.L., Watson R.E., Patel B.A., Gulbransen B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2016;2(1):77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClain J., Grubišić V., Fried D., Gomez-Suarez R.A., Leinninger G.M., Sévigny J., Parpura V., Gulbransen B.D. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterol. 2014;146(2):497–507.e1. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coelho-Aguiar Jde M., Bon-Frauches A.C., Gomes A.L., Veríssimo C.P., Aguiar D.P., Matias D., Thomasi B.B., Gomes A.S., Brito G.A., Moura-Neto V. The enteric glia: identity and functions. Glia. 2015;63(6):921–935. doi: 10.1002/glia.22795. [DOI] [PubMed] [Google Scholar]
- 40.Rao M., Nelms B.D., Dong L., Salinas-Rios V., Rutlin M., Gershon M.D., Corfas G. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia. 2015;63(11):2040–2057. doi: 10.1002/glia.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vainchtein I.D., Chin G., Cho F.S., Kelley K.W., Miller J.G., Chien E.C., Liddelow S.A., Nguyen P.T., Nakao-Inoue H., Dorman L.C., Akil O., Joshita S., Barres B.A., Paz J.T., Molofsky A.B., Molofsky A.V. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359(6381):1269–1273. doi: 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Luca C., Savarese L., Colangelo A.M., Bianco M.R., Cirillo G., Alberghina L., Papa M. Astrocytes and microglia-mediated immune response in maladaptive plasticity is differently modulated by NGF in the ventral horn of the spinal cord following peripheral nerve injury. Cell. Mol. Neurobiol. 2016;36(1):37–46. doi: 10.1007/s10571-015-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dogiel A.S. Ueber den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugethiere. Arch Anat Physiol Anat Abt (Leipzig) 1899:130–158. [Google Scholar]
- 45.Gabella G. Glial cells in the myenteric plexus. Z Naturforsch B. 1971;26(3):244–245. doi: 10.1515/znb-1971-0313. [DOI] [PubMed] [Google Scholar]
- 46.Delvalle N.M., Dharshika C., Morales-Soto W., Fried D.E., Gaudette L., Gulbransen B.D. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell. Mol. Gastroenterol. Hepatol. 2018;6(3):321–344. doi: 10.1016/j.jcmgh.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Boyen G.B., Steinkamp M., Reinshagen M., Schäfer K.H., Adler G., Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004 Feb;53(2):222–228. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63(8):1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 49.Brown I.A., McClain J.L., Watson R.E., Patel B.A., Gulbransen B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2016;2(1):77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grubišić V., McClain J.L., Fried D.E., Grants I., Rajasekhar P., Csizmadia E., Ajijola O.A., Watson R.E., Poole D.P., Robson S.C., Christofi F.L., Gulbransen B.D. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell. Rep. 2020;32(10):108100. doi: 10.1016/j.celrep.2020.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoffels B., Hupa K.J., Snoek S.A., van Bree S., Stein K., Schwandt T., Vilz T.O., Lysson M., Veer C.V., Kummer M.P., Hornung V., Kalff J.C., de Jonge W.J., Wehner S. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology. 2014 Jan;146(1):176. doi: 10.1053/j.gastro.2013.09.030. 87.e1. [DOI] [PubMed] [Google Scholar]
- 52.Stakenborg Michelle, Abdurahiman Saeed, De Simone Veronica, Goverse Gera, Stakenborg Nathalie, van Baarle Lies, Wu Qin, Pirottin Dimitri, Kim Jung-Seok, Chappell-Maor Louise, Pintelon Isabel, Thys Sofie, Boon Louis, Marlene Hao, Jo A., Van Ginderachter, Boeckxstaens Guy E., Timmermans Jean- Pierre, Jung Steffen, Marichal Thomas, Ibiza Sales, Matteoli Gianluca. Enteric glial cells favour accumulation of anti-inflammatory macrophages during the resolution of muscularis inflammation. bioRxiv. 2021;06(10):447700. doi: 10.1101/2021.06.10.447700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Progatzky F., Shapiro M., Chng S.H., Garcia-Cassani B., Classon C.H., Sevgi S., Laddach A., Bon-Frauches A.C., Lasrado R., Rahim M., Amaniti E.M., Boeing S., Shah K., Entwistle L.J., Suárez-Bonnet A., Wilson M.S., Stockinger B., Pachnis V. Regulation of intestinal immunity and tissue repair by enteric glia. Nature. 2021 Nov;599(7883):125–130. doi: 10.1038/s41586-021-04006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Challis C., Hori A., Sampson T.R., Yoo B.B., Challis R.C., Hamilton A.M., Mazmanian S.K., Volpicelli-Daley L.A., Gradinaru V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci. 2020 Mar;23(3):327–336. doi: 10.1038/s41593-020-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z., Chen S., Qiu C., Sun Y., Li W., Jiang J., Zhang J.M. Fractalkine/CX3CR1 Contributes to endometriosis-induced neuropathic pain and mechanical hypersensitivity in rats. Front. Cell. Neurosci. 2018;12:495. doi: 10.3389/fncel.2018.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolós M., Perea J.R., Terreros-Roncal J., Pallas-Bazarra N., Jurado-Arjona J., Ávila J., Llorens-Martín M. Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav. Immun. 2018;68:76–89. doi: 10.1016/j.bbi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Bachiller S., Jiménez-Ferrer I., Paulus A., Yang Y., Swanberg M., Deierborg T., Boza-Serrano A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L., Li Q., Saha M., Li C., Enikolopov G., Becker L., Rakhilin N., Anderson M., Shen X., Dong X., Butte M.J., Song H., Southard-Smith E.M., Kapur R.P., Bogunovic M., Pasricha P.J. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2017;114(18):E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cipriani G., Terhaar M.L., Eisenman S.T., Ji S., Linden D.R., Wright A.M., Sha L., Ordog T., Szurszewski J.H., Gibbons S.J., Farrugia G. Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell. Mol. Gastroenterol. Hepatol. 2019;7(3):689–691. doi: 10.1016/j.jcmgh.2019.01.005. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker L., Spear E.T., Sinha S.R., Haileselassie Y., Habtezion A. Age-related changes in gut microbiota alter phenotype of muscularis macrophages and disrupt gastrointestinal motility. Cell. Mol. Gastroenterol. Hepatol. 2019;7(1):243–245. doi: 10.1016/j.jcmgh.2018.09.001. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker L., Nguyen L., Gill J., Kulkarni S., Pasricha P.J., Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67(5):827–836. doi: 10.1136/gutjnl-2016-312940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graves C.L., Chen A., Kwon V., Shiau C.E. Zebrafish harbor diverse intestinal macrophage populations including a subset intimately associated with enteric neural processes. iScience. 2021 May 3;24(6):102496. doi: 10.1016/j.isci.2021.102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf Y.S., Boura-Halfon N., Cortese Z., Haimon H., Sar Shalom Y., Kuperman V., Kalchenko A., Brandis E., David Y., Segal-Hayoun Y., Chappell-Maor L., Yaron A., Jung S. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 2017;18:665–674. doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolter J.R., Feuerstein P., Zeis N., Hagemeyer N., Paterson P., d’Errico S., Baasch L., Amann T., Masuda A., Lösslein A., Gharun K., Meyer-Luehmann M., Waskow C., Franzke C.W., Grün D., Lämmermann T., Prinz M., Henneke P. A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity. 2019;50:1482–1497. doi: 10.1016/j.immuni.2019.05.009. e7. [DOI] [PubMed] [Google Scholar]
- 65.Phillips R.J., Powley T.L. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton. Neurosci. 2012;169(1):12–27. doi: 10.1016/j.autneu.2012.02.004. Epub 2012 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matheis F., Muller P.A., Graves C.L., Gabanyi I., Kerner Z.J., Costa-Borges D., Ahrends T., Rosenstiel P., Mucida D. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell. 2020;180(1):64–78. doi: 10.1016/j.cell.2019.12.002. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 68.Osborne-Hereford A.V., Rogers S.W., Gahring L.C. Neuronal nicotinic alpha7 receptors modulate inflammatory cytokine production in the skin following ultraviolet radiation. J. Neuroimmunol. 2008;193(1-2):130–139. doi: 10.1016/j.jneuroim.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matteoli G., Boeckxstaens G.E. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62(8):1214–1222. doi: 10.1136/gutjnl-2012-302550. Epub 2012 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matteoli G., Gomez-Pinilla P.J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S.H., Michel K., Tracey K.J., Schemann M., Boesmans W., Vanden Berghe P., Boeckxstaens G.E. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 71.Yuan P.Q., Taché Y. Abdominal surgery induced gastric ileus and activation of M1-like macrophages in the gastric myenteric plexus: prevention by central vagal activation in rats. Am. J. Physiol .Gastrointest. Liver Physiol. 2017;313(4):G320–G329. doi: 10.1152/ajpgi.00121.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cserép C., Pósfai B., Lénárt N., Fekete R., László Z.I., Lele Z., Orsolits B., Molnár G., Heindl S., Schwarcz A.D., Ujvári K., Környei Z., Tóth K., Szabadits E., Sperlágh B., Baranyi M., Csiba L., Hortobágyi T., Maglóczky Z., Martinecz B., Szabó G., Erdélyi F., Szipőcs R., Tamkun M.M., Gesierich B., Duering M., Katona I., Liesz A., Tamás G., Dénes Á. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367(6477):528–537. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 73.Sanders K.M., Stevens R., Burke E., Ward S.W. Slow waves actively propagate at submucosal surface of circular layer in canine colon. Am. J. Physiol. 1990;259(2 Pt 1):G258–G263. doi: 10.1152/ajpgi.1990.259.2.G258. [DOI] [PubMed] [Google Scholar]
- 74.Dickens E.J., Hirst G.D., Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J. Physiol. 1999;514(Pt 2):515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. (Pt 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lies B., Gil V., Groneberg D., Seidler B., Saur D., Wischmeyer E., Jiménez M., Friebe A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307(1):G98–106. doi: 10.1152/ajpgi.00082.2014. [DOI] [PubMed] [Google Scholar]
- 76.Ward S.M., Sanders K.M. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J. Physiol. 2006;576(Pt 3):675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burns A.J., Lomax A.E., Torihashi S., Sanders K.M., Ward S.M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl. Acad. Sci. U. S. A. 1996;93(21):12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hulsmans M., Clauss S., Xiao L., Aguirre A.D., King K.R., Hanley A., Hucker W.J., Wülfers E.M., Seemann G., Courties G., Iwamoto Y., Sun Y., Savol A.J., Sager H.B., Lavine K.J., Fishbein G.A., Capen D.E., Da Silva N., Miquerol L., Wakimoto H., Seidman C.E., Seidman J.G., Sadreyev R.I., Naxerova K., Mitchell R.N., Brown D., Libby P., Weissleder R., Swirski F.K., Kohl P., Vinegoni C., Milan D.J., Ellinor P.T., Nahrendorf M. Macrophages facilitate electrical conduction in theh. Cell. 2017;169(3):510–522. doi: 10.1016/j.cell.2017.03.050. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., Kong P., Chen C., Tang J., Jin X., Yan J., Wang Y. Targeting IL-17A Improves the dysmotility of the small intestine and alleviates the injury of the interstitial cells of Cajal during sepsis. Oxid. Med. Cell. Longev. 2019;Aug 18:1475729. doi: 10.1155/2019/1475729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X.Y., Berezin I., Mikkelsen H.B., Der T., Bercik P., Collins S.M., Huizinga J.D. Pathology of interstitial cells of Cajal in relation to inflammation revealed by ultrastructure but not immunohistochemistry. Am. J. Pathol. 2002;160(4):1529–1540. doi: 10.1016/s0002-9440(10)62579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avetisyan M., Rood J.E., Huerta Lopez S., Sengupta R., Wright-Jin E., Dougherty J.D., Behrens E.M., Heuckeroth R.O. Muscularis macrophage development in the absence of an enteric nervous system. Proc. Natl. Acad. Sci. U. S. A. 2018;115(18):4696–4701. doi: 10.1073/pnas.1802490115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng J., Yang S., Yuan Q., Chen Y., Li D., Sun H., Tan X., Zhang F., Zhou D. Acupuncture ameliorates postoperative ileus via IL-6-miR-19a-KIT axis to protect interstitial cells of Cajal. Am. J. Chin. Med. 2017;45:737–755. doi: 10.1142/S0192415X17500392. [DOI] [PubMed] [Google Scholar]
- 83.Chen X., Meng X., Zhang H., Feng C., Wang B., Li N., Abdullahi K.M., Wu X., Yang J., Li Z., Jiao C., Wei J., Xiong X., Fu K., Yu L., Besner G.E., Feng J. Intestinal proinflammatory macrophages induce a phenotypic switch in interstitial cells of Cajal. J. Clin. Invest. 2020;130(12):6443–6456. doi: 10.1172/JCI126584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eisenman S.T., Gibbons S.J., Verhulst P.J., Cipriani G., Saur D., Farrugia G. Tumor necrosis factor alpha derived from classically activated “M1” macrophages reduces interstitial cell of Cajal numbers. Neurogastroenterol Motil. 2017 Apr;29(4) doi: 10.1111/nmo.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grover M., Bernard C.E., Pasricha P.J., Parkman H.P., Gibbons S.J., Tonascia J., Koch K.L., McCallum R.W., Sarosiek I., Hasler W.L., Nguyen L.A.B., Abell T.L., Snape W.J., Kendrick M.L., Kellogg T.A., McKenzie T.J., Hamilton F.A., Farrugia G., NIDDK Gastroparesis Clinical Research Consortium (GpCRC) Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol. Motil. 2017;29(6) doi: 10.1111/nmo.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernard C.E., Gibbons S.J., Mann I.S., Froschauer L., Parkman H.P., Harbison S., Abell T.L., Snape W.J., Hasler W.L., McCallum R.W., Sarosiek I., Nguyen L.A., Koch K.L., Tonascia J., Hamilton F.A., Kendrick M.L., Shen K.R., Pasricha P.J., Farrugia G., NIDDK Gastroparesis Clinical Research Consortium (GpCRC) Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol. Motil. 2014;26(9):1275–1284. doi: 10.1111/nmo.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kashyap P.C., Choi K.M., Dutta N., Linden D.R., Szurszewski J.H., Gibbons S.J., Farrugia G. Carbon monoxide reverses diabetic gastroparesis in NOD mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298(6):G1013–G1019. doi: 10.1152/ajpgi.00069.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi K.M., Gibbons S.J., Sha L., Beyder A., Verhulst P.J., Cipriani G., Phillips J.E., Bauer A.J., Ordog T., Camp J.J., Ge X., Bharucha A.E., Linden D.R., Szurszewski J.H., Kashyap P.C., Farrugia G. Interleukin 10 restores gastric emptying, electrical activity, and interstitial cells of Cajal networks in diabetic mice. Cell. Mol. Gastroenterol Hepatol. 2016;2(4):454–467. doi: 10.1016/j.jcmgh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X.Y., Zarate N., Soderholm J.D., Bourgeois J.M., Liu L.W., Huizinga J.D. Ultrastructural injury to interstitial cells of Cajal and communication with mast cells in Crohn's disease. Neurogastroenterol. Motil. 2007;19:349–364. doi: 10.1111/j.1365-2982.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 90.Zarate N., Wang X.Y., Tougas G., Anvari M., Birch D., Mearin F., Malagelada J.R., Huizinga J.D. Intramuscular interstitial cells of Cajal associated with mast cells survive nitrergic nerves in achalasia. Neurogastroenterol. Motil. 2006;18:556–568. doi: 10.1111/j.1365-2982.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki T., Won K.J., Horiguchi K., Kinoshita K., Hori M., Torihashi S., Momotani E., Itoh K., Hirayama K., Ward S.M., Sanders K.M., Ozaki H. Muscularis inflammation and the loss of interstitial cells of Cajal in the endothelin ETB receptor null rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G638–G646. doi: 10.1152/ajpgi.00077.2004. [DOI] [PubMed] [Google Scholar]
- 92.Lee M.J., Kim M.Y., Heo S.C., Kwon Y.W., Kim Y.M., Do E.K., Park J.H., Lee J.S., Han J., Kim J.H. Macrophages regulate smooth muscle differentiation of mesenchymal stem cells via a prostaglandin F₂α-mediated paracrine mechanism. Arterioscler. Thromb. Vasc. Biol. 2012;32(11):2733–2740. doi: 10.1161/ATVBAHA.112.300230. [DOI] [PubMed] [Google Scholar]
- 93.Luo J., Qian A., Oetjen L.K., Yu W., Yang P., Feng J., Xie Z., Liu S., Yin S., Dryn D., Cheng J., Riehl T.E., Zholos A.V., Stenson W.F., Kim B.S., Hu H. TRPV4 Channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity. 2018;49(1):107–119. doi: 10.1016/j.immuni.2018.04.021. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hori M., Kita M., Torihashi S., Miyamoto S., Won K.J., Sato K., Ozaki H., Karaki H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280(5):G930–G938. doi: 10.1152/ajpgi.2001.280.5.G930. [DOI] [PubMed] [Google Scholar]
- 95.Eskandari M.K., Kalff J.C., Billiar T.R., Lee K.K., Bauer A.J. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am. J. Physiol. 1997;273(3 Pt 1):G727–G734. doi: 10.1152/ajpgi.1997.273.3.G727. [DOI] [PubMed] [Google Scholar]
- 96.Hori M., Nobe H., Horiguchi K., Ozaki H. MCP-1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am. J. Physiol. Cell. Physiol. 2008;294(2):C391–401. doi: 10.1152/ajpcell.00056.2007. [DOI] [PubMed] [Google Scholar]
- 97.Ohama T., Hori M., Momotani E., Elorza M., Gerthoffer W.T., Ozaki H. IL-1beta inhibits intestinal smooth muscle proliferation in an organ culture system: involvement of COX-2 and iNOS induction in muscularis resident macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(5):G1315–G1322. doi: 10.1152/ajpgi.00487.2006. [DOI] [PubMed] [Google Scholar]
- 98.Komuro T., Seki K., Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch. Histol. Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- 99.Lino S., Horiguchi K., Horiguchi S., Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor α in the murine gastrointestinal musculature. Histochem. Cell. Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 100.Kurahashi M., Zheng H., Dwyer L., Ward S.M., Koh S.D., Sanders K.M. A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles. J. Physiol. 2011;589(Pt 3):697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horiguchi K., Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J. Auton. Nerv. Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 102.Bischoff S.C., Wedemeyer J., Herrmann A., Meier P.N., Trautwein C., Cetin Y., Maschek H., Stolte M., Gebel M., Manns M.P. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28:1–13. doi: 10.1046/j.1365-2559.1996.262309.x. [DOI] [PubMed] [Google Scholar]
- 103.Shea-Donohue T., Urban J.F., Jr. Neuroimmune Modulation of Gut Function. Handb Exp Pharmacol. 2017;239:247–267. doi: 10.1007/164_2016_109. [DOI] [PubMed] [Google Scholar]
- 104.Boeckxstaens G.E. The emerging role of mast cells in irritable bowel syndrome. Gastroenterol Hepatol (NY) 2018;14:250–252. [PMC free article] [PubMed] [Google Scholar]
- 105.Wouters M.M., VicarioM Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 106.McClain J.L., Mazzotta E.A., Maradiaga N., Duque-Wilckens N., Grants I., Robison A.J., Christofi F.L., Moeser A.J., Gulbransen B.D. Histamine-dependent interactions between mast cells, glia, and neurons are altered following early-life adversity in mice and humans. Am J Physiol Gastrointest Liver Physiol. 2020 Dec 1;319(6):G655–G668. doi: 10.1152/ajpgi.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buhner S., Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. 2012;1822(1):85–92. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Schemann M., Camilleri M. Functions and imaging of mast cell and neural axis of the gut. Gastroenterology. 2013 Apr;144(4):698–704. doi: 10.1053/j.gastro.2013.01.040. Epub 2013 Jan 24. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stead R.H., Dixon M.F., Bramwell N.H., Riddell R.H., Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- 110.Stead R.H. Innervation of mucosal immune cells in the gastrointestinal tract. Reg. Immunol. 1992;4:91–99. [PubMed] [Google Scholar]
- 111.Chesné J., Cardoso V., Veiga-Fernandes H. Neuro-immune regulation of mucosal physiology. Mucosal Immunol. 2019 Jan;12(1):10–20. doi: 10.1038/s41385-018-0063-y. Epub 2018 Aug 8. PMID: 30089849. [DOI] [PubMed] [Google Scholar]
- 112.Wang G.D., Wang X.Y., Liu S., Qu M., Xia Y., Needleman B.J., Mikami D.J., Wood J.D. Innervation of enteric mast cells by primary spinal afferents in guinea pig and human small intestine. Am J Physiol Gastrointest Liver Physiol. 2014 Oct 1;307(7):G719–G731. doi: 10.1152/ajpgi.00125.2014. Epub 2014 Aug 21. PMID: 25147231; PMCID: PMC4187066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shalit M., Brenner T., Shohami E., Levi-Schaffer F. Interaction between mast cells and glial cells: an in vitro study. J Neuroimmunol. 1993 Mar;43(1-2):195–199. doi: 10.1016/0165-5728(93)90092-d. [DOI] [PubMed] [Google Scholar]
- 114.Kritas S.K., Saggini A., Cerulli G., Caraffa A., Antinolfi P., Pantalone A., Saggini R., Frydas S., Rosati M., Tei M., Speziali A., Pandolfi F., Conti P. Interrelationship between IL-3 and mast cells. J Biol Regul Homeost Agents. 2014 Jan-Mar;28(1):17–21. PMID: 24750787. [PubMed] [Google Scholar]
- 115.Bradford K., Shih W., Videlock E.J., Presson A.P., Naliboff B.D., Maye E.A., Chang L. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. doi: 10.1016/j.cgh.2011.12.018. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He C.L., Soffer E.E., Ferris C.D., et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 117.Rumessen J.J. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology. 1996;111:1447–1455. doi: 10.1016/s0016-5085(96)70005-7. [DOI] [PubMed] [Google Scholar]
- 118.Coldwell J.R., Phillis B.D., Sutherland K., Howarth G.S., Blackshaw L.A. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol. 2007;579(Pt 1):203–213. doi: 10.1113/jphysiol.2006.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ravanbakhsh N., Kesavan A. The role of mast cells in pediatric gastrointestinal disease. Ann Gastroenterol. 2019;32(4):338–345. doi: 10.20524/aog.2019.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meira de-Faria F., Casado-Bedmar M., Mårten Lindqvist C., Jones M.P., Walter S.A., Keita Å.V. Altered interaction between enteric glial cells and mast cells in the colon of women with irritable bowel syndrome. Neurogastroenterol Motil. 2021;2 doi: 10.1111/nmo.14130. [DOI] [PubMed] [Google Scholar]
- 121.Lakhan S.E., Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. 2010;8:7–37. doi: 10.1186/1742-2094-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fukumoto Y., Kasai H., Takahashi H., Sugiyama H., Hase N., Kaneko H., Hamamura I., Aoki Y., Ota M., Kobayashi T., Katsuura Y., Kamimura T., Komoriya K. The role of mast cells in the development of 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in rats. Scand J Gastroenterol. 2002;37(5):555–560. doi: 10.1080/00365520252903107. [DOI] [PubMed] [Google Scholar]
- 123.Xu X., Weksler-Zangen S., Pikarsky A., Pappo O., Wengrower D., Bischoff S.C., Pines M., Rivkind A., Goldin E., Levi-Schaffer F. Mast cells involvement in the inflammation and fibrosis development of the TNBS-induced rat model of colitis. Scand J Gastroenterol. 2002;37(3):330–337. doi: 10.1080/003655202317284246. [DOI] [PubMed] [Google Scholar]
- 124.Xu X., Weksler-Zangen S., Pikarsky A., Pappo O., Wengrower D., Bischoff S.C., Pines M., Rivkind A., Goldin E., Levi-Schaffer F. Mast cells involvement in the inflammation and fibrosis development of the TNBS-induced rat model of colitis. Scand J Gastroenterol. 2002;37(3):330–337. doi: 10.1080/003655202317284246. [DOI] [PubMed] [Google Scholar]
- 125.Chichlowski M., Westwood G.S., Abraham S.N., Hale L.P. Role of mast cells in inflammatory bowel disease and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mazzotta E., Villalobos-Hernandez E.C., Fiorda-Diaz J., Harzman A., Christofi F.L. Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.583422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kalff J.C., Carlos T.M., Schraut W.H., Billiar T.R., Simmons R.L., Bauer A.J. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117(2):378–387. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- 128.Li Y.Y., Cao M.H., Goetz B., Chen C.Q., Feng Y.J., Chen C.J., Kasparek M.S., Sibaev A., Storr M., Kreis M.E. The dual effect of cannabinoid receptor-1 deficiency on the murine postoperative ileus. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Jonge W.J., The F.O., van der Coelen D., Bennink R.J., Reitsma P.H., van Deventer S.J., van den Wijngaard R.M., Boeckxstaens G.E. Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice. Gastroenterology. 2004;127(2):535–545. doi: 10.1053/j.gastro.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 130.Gomez-Pinilla P.J., Farro G., Di Giovangiulio M., Stakenborg N., Némethova A., de Vries A., Liston A., Feyerabend T.B., Rodewald H.R., Boeckxstaens G.E., Matteoli G. Mast cells play no role in the pathogenesis of postoperative ileus induced by intestinal manipulation. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.The F.O., Bennink R.J., Ankum W.M., Buist M.R., Busch O.R., Gouma D.J., van der Heide S., van den Wijngaard R.M., de Jonge W.J., Boeckxstaens G.E. Intestinal handling-induced mast cell activation and inflammation in human postoperative ileus. Gut. 2008;57(1):33–40. doi: 10.1136/gut.2007.120238. [DOI] [PubMed] [Google Scholar]
- 132.Liu Z.Q., Chen W.F., Wang Y., Xu X.Y., Zeng Y.G., Lee Dillon D., Cheng J., Xu M.D., Zhong Y.S., Zhang Y.Q., Yao L.Q., Zhou P.H., Li Q.L. Mast cell infiltration associated with loss of interstitial cells of Cajal and neuronal degeneration in achalasia. Neurogastroenterol Motil. 2019;31(5):e13565. doi: 10.1111/nmo.13565. [DOI] [PubMed] [Google Scholar]
- 133.Nelson M., Zhang X., Genta R.M., Turner K., Podgaetz E., Paris S., Cardenas J., Gu J., Leeds S., Ward M., Nguyen A., Konda V., Furuta G.T., Pan Z., Souza R.F., Spechler S.J. Lower esophageal sphincter muscle of patients with achalasia exhibits profound mast cell degranulation. Neurogastroenterol Motil. 2021;33(5) doi: 10.1111/nmo.14055. [DOI] [PubMed] [Google Scholar]
- 134.Yadav A.K., Mishra K., Mohta A., Agarwal S. Hirschsprung's disease: is there a relationship between mast cells and nerve fibers? World J Gastroenterol. 2009;15(12):1493–1498. doi: 10.3748/wjg.15.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]