Abstract
Introduction
Despite the availability of vaccines, pneumococcal disease (PD) is associated with high clinical and economic burden, mainly caused by non-vaccine serotypes and certain vaccine-type serotypes. V114 is a 15-valent pneumococcal conjugate vaccine (PCV) that contains two epidemiologically important serotypes, 22F and 33F, in addition to the 13 serotypes in 13-valent PCV (PCV13). This study quantified the epidemiologic and economic burden of PD attributable to V114 serotypes among adults in the USA.
Methods
A Markov model was used to estimate the burden of V114 serotypes in a hypothetical, non-vaccinated cohort of US adults ≥ 19 years of age who were tracked from 2019 until death. The model calculated all the invasive pneumococcal disease (IPD) and non-bacteremic pneumococcal pneumonia (NBPP) cases, deaths, and costs. Economic burden was estimated from a healthcare payer perspective (2019 US dollars) and discounted at 3%.
Results
The model estimated 415,229 and 10.3 million lifetime cases of V114-type IPD and NBPP, respectively. Serotypes 22F and 33F caused approximately 33% of IPD cases and 20% of NBPP cases, while serotype 3 accounted for approximately 36% of IPD cases and 13% of NBPP cases. V114 serotypes caused 472,063 total lifetime deaths. Total discounted lifetime costs attributable to V114 serotypes were $44.8 billion US dollars.
Conclusions
In this hypothetical model of a non-vaccinated cohort of US adults, V114 serotypes were associated with a substantial health and economic burden, the majority of which was attributable to serotypes 3, 22F, and 33F. The addition of V114 to the national vaccination recommendations may help to reduce the epidemiologic and economic burden associated with PD in adults ≥ 19 years of age in the USA by providing increased coverage of these serotypes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00588-x.
Keywords: Invasive pneumococcal disease, Non-bacteremic pneumococcal pneumonia, Pneumococcal conjugate vaccine, Markov model, Health and economic burden
Key Summary Points
| Why carry out this study? |
| Pneumococcal disease (PD) is associated with significant morbidity, mortality, and costs among adults in the USA. |
| V114 is a 15-valent pneumococcal conjugate vaccine that contains two epidemiologically important serotypes, 22F and 33F, in addition to the 13 serotypes in the 13-valent pneumococcal conjugate vaccine. |
| In this study, we aimed to estimate the epidemiologic and economic burden of PD attributable to V114 serotypes among adults in the USA. |
| What was learned from the study? |
| Our results suggest a substantial burden of PD caused by V114 serotypes, especially serotypes 3, 22F, and 33F. |
| The introduction of V114 into the national immunization schedule may help reduce the burden caused by V114 serotypes in US adults. |
Introduction
Streptococcus pneumoniae is a Gram-positive bacterium that commonly colonizes the respiratory tract, potentially causing invasive and non-invasive pneumococcal disease (PD) [1]. Pneumococcal pneumonia is the most common clinical presentation of PD among adults in the USA [1]. The common types of invasive pneumococcal disease (IPD) include bacteremic pneumonia, meningitis, and septicemia, whereas non-invasive disease comprises pneumonia without bacteremia, otitis media, and sinusitis [1]. The annual incidence of IPD in US adults 19–64 years of age and adults ≥ 65 years of age was eight cases per 100,000 and 24 cases per 100,000 in 2018, respectively [2]. Non-bacteremic pneumococcal pneumonia (NBPP) continues to be a major concern, accounting for approximately 75% of cases of pneumococcal pneumonia [3]. Moreover, the presence of comorbid conditions is associated with a higher risk of PD [4, 5].
PD continues to cause significant morbidity and mortality in the USA. In 2004, PD was estimated to be responsible for 4 million episodes of illness, 445,000 hospitalizations, and 22,000 deaths in the USA [6]. The Active Bacterial Core surveillance (ABCs) report for S. pneumoniae from 2018 estimated 3297 cases and 361 deaths per 100,000 population in the USA; a high mortality rate was observed among adults > 35 years of age in the USA, with higher rates seen in individuals ≥ 65 years of age [7]. PD is also associated with high healthcare costs [6, 8]. In 2015, the total US costs of PD were estimated at $1.86 billion in adults ≥ 19 years of age, of which $1.8 billion was attributable to direct medical costs [8].
The introduction of a 7-valent pneumococcal conjugate vaccine (PCV7 or Prevnar®, Wyeth Pharmaceuticals Inc. Philadelphia, PA, USA) in 2000 to pediatric immunization programs in the USA, and its replacement in 2010 with a 13-valent pneumococcal conjugate vaccine (PCV13 or Prevnar 13®, Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc., Philadelphia, PA, USA), has been associated with reduced carriage and transmission of S. pneumoniae among non-vaccinated infants and a substantial decline in the incidence of vaccine-type IPD among adults ≥ 65 years of age through indirect effects of PCV13 use [9]. The use of PCV13 was recommended for all adults ≥ 65 years of age in 2014; however, the reduction in cases has since plateaued [2, 9] and IPD has continued to cause significant morbidity and mortality in this age group [7]. Pneumococcal vaccine coverage in the USA in 2018 was 23.3% and 69% among adults 19–64 years of age and ≥ 65 years of age, respectively [10]. In 2019, the US Advisory Committee on Immunization Practices recommendation for routine PCV13 vaccination was updated to shared clinical decision-making for PCV13 use in adults ≥ 65 years of age without an immunocompromising condition [9]. The sustained prevalence of NBPP and IPD may be related to strains not covered by PCV13. A considerable proportion of cases of IPD in the USA are caused by the non-PCV13 serotypes 22F and 33F, which are among the most common causes of IPD in adults ≥ 18 years of age [11, 12]. In addition, certain PCV13 vaccine serotypes, such as serotype 3, continue to impose a considerable burden [12, 13].
The US Food and Drug Administration recently approved a 15-valent pneumococcal conjugate vaccine, V114 (VAXNEUVANCE™, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA), for active immunization to prevent/reduce invasive disease caused by S. pneumoniae in adults ≥ 18 years of age in the USA. V114 contains antigens for serotypes 22F and 33F, along with the 13 serotypes included in PCV13 [14, 15].
The objective of this study was to quantify the epidemiologic and economic burden of PD attributable to the 15 serotypes targeted by V114 in a hypothetical, non-vaccinated cohort in the USA.
Methods
Analytical Approach
A probabilistic Markov state transition model was used to conduct this analysis from a healthcare payer perspective.
Model Overview
A state-transition global Markov model was developed to track adults until death or 100 years of age. The adult population was divided into cohorts of low-risk, at-risk (high-risk but immunocompetent), and high-risk immunosuppressed adults.
Model Structure
As illustrated in Fig. 1, the model contains six health states: no PD, inpatient NBPP, outpatient NBPP, IPD, post meningitis sequelae (PMS), and death. All individuals enter the model in the “no PD” health state and are subsequently at risk of developing IPD or NBPP. Vaccinated individuals without PD have a lower chance of developing IPD or NBPP. Individuals who develop IPD or NBPP are faced with a higher risk of death than those without PD. After IPD or NBPP, surviving individuals either recover and return to the “no PD” health state or may develop PMS after IPD meningitis. It is assumed that, after PMS, the patients cannot develop another episode of IPD or NBPP, because such a situation is considered rare. Hence, the PMS health state is an absorbing health state, and they may subsequently die. However, the background mortality still applies to the individuals with PMS. The model tracks the cohort over the specified time horizon and calculates all the IPD and NBPP cases, deaths, costs, and quality-adjusted life years (QALYs); not all of these data are included in this report. In addition, half-cycle correction is applied to account for events that occur at any point (week/month) during the year.
Fig. 1.
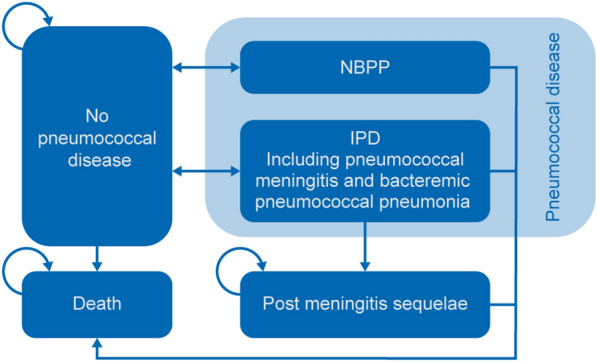
Markov model structure. IPD invasive pneumococcal disease, NBPP non-bacteremic pneumococcal pneumonia. Background mortality is applied to all health states in the model
Model Inputs
The model has the following input categories:
General model inputs (currency, perspective, time horizon, uncertainty assumptions, and discount rate).
Population inputs (population size, life table, proportion of patients in different risk groups, age of vaccine administrations, choice of intervention and comparators, and vaccine coverage rate).
Efficacy inputs (serotype distribution together with serotype-specific vaccine efficacy for intervention and comparators, vaccine waning function, childhood herd immunity, and childhood serotype replacement).
Epidemiological inputs (proportion of antimicrobial resistance per serotype, IPD and NBPP incidence, case fatality rate [CFR] for IPD and NBPP, proportion of meningitis in IPD cases, proportion of PMS in meningitis cases and proportion of NBPP cases hospitalized).
Cost inputs (vaccine and vaccine administration costs, costs of IPD, inpatient and outpatient NBPP, meningitis, PMS, and antimicrobial resistance cases, loss of productivity costs, and travel costs).
Utility inputs (baseline utility value per risk group and age group, utility decrement values of PD, utility value of individuals with PMS, and days of decrement per PD). All input values are modifiable and can be updated with data for the population under study.
The outputs generated by the model can include the number of cases of IPD, inpatient and outpatient NBPP, PMS, and antimicrobial resistance cases in IPD and NBPP for each vaccination strategy, by subgroup and by serotype within each subgroup. Other outcomes that can be determined by the model include IPD and NBPP deaths, serotype-specific costs, incremental costs, life years and QALYs, incremental cost-effectiveness ratios, sensitivity analyses, and threshold analyses. The lifetime horizon is chosen to capture all benefits provided by the vaccinations throughout the study population’s lifetime. The cost and outcomes can be discounted at separate adaptable rates in the model. The model uses a 1-year cycle length to circumvent the seasonality of PD [16].
Model Validation
The model implementation has been verified through a series of tests to ensure that the model is performing as expected and is in line with the design objective, and that the outputs of the model are acceptable with respect to the real data-generating process. The technical aspects were checked against a checklist, stress testing was applied to the model, and tests were built into the model for verification and to ensure internal validity [17]. Finally, the model results were checked with the systematic literature review outcomes of the study by Shiri and colleagues [18].
The Current Analysis
Given the research question and objective of the current analysis (quantifying the epidemiologic and economic burden of PD attributable to the 15 serotypes targeted by V114 in a hypothetical, non-vaccinated cohort in the USA), the model was used to predict the number of cases and associated costs for the 15 serotypes over the lifetime of all adults (≥ 19 years of age) in the USA.
This article does not contain any new studies with human participants or animals performed by any of the authors. The data used are presented below.
Target Population
The target population of the study was a 2019 cohort of non-vaccinated adults in the USA ≥ 19 years of age who were tracked from 2019 until death. The cohort size of approximately 250 million adults was based on annual estimates of the adult population in the USA in 2019 according to the US Census Bureau [19]. The study population was stratified into three mutually exclusive risk groups: low-risk (LR), at-risk (AR), and high-risk (HR) adults, based on proportions per age group (Table S1 in the supplementary material) from Pelton et al. [4].
LR refers to healthy individuals.
AR refers to immunocompetent individuals having one or more of the following conditions: chronic heart disease, diabetes mellitus, pulmonary disease, liver disease, alcoholism, cerebrospinal fluid leaks, cochlear implants, or cigarette smoking/smokers.
HR refers to immunosuppressed individuals who have one of the following conditions: anatomic asplenia, human immunodeficiency virus infection, leukemia, lymphoma, Hodgkin disease, multiple myeloma, generalized malignancy, chronic renal failure, nephrotic syndrome, or other conditions associated with immunosuppression (e.g., organ or bone marrow transplantation).
Having more than one of these conditions is not considered in the model. The model combines all conditions related to high-risk immunosuppression into one high-risk immunosuppressed subgroup. The model assumes that changes between different risk groups (e.g., from LR to AR following the incidence of a medical condition, such as diabetes mellitus) are not possible.
Life Table
A life table presenting the age-specific annual probabilities of death based on the general population was included to cover background mortality for the cohort (Table S2 in supplementary material). These probabilities were applied to the model population to ensure that the natural survival seen in the general population is reflected. The life table was obtained from the US National Vital Statistics Report [20].
Epidemiological Inputs
Epidemiological data obtained from published literature and publicly available reports were used as model parameters (Table 1). IPD incidence was obtained from Ahmed et al. [21] and serotype distribution was obtained from unpublished 2017 CDC serotype data shared with the authors. The CFRs for IPD were taken from the ABCs report for S. pneumoniae from 2017 [22].
Table 1.
Epidemiologic model parameters
| Model parameters | 18–64 years | 65+ years | ||||
|---|---|---|---|---|---|---|
| LR | AR | HR | LR | AR | HR | |
|
IPD incidence (per 100,000) [21] |
3.90 | 14.00 | 26.60 | 15.40 | 35.40 | 38.70 |
| Proportion of ST3 IPD (%)† | 12.10 | 12.10 | 12.10 | 15.5 | 15.5 | 15.5 |
| Proportion of 22F + 33F IPD (%)† | 11.20 | 11.20 | 11.20 | 13.20 | 13.20 | 13.20 |
| Proportion of V114 serotypes (%)† | 35.10 | 35.10 | 35.10 | 36.60 | 36.60 | 36.60 |
| Case fatality rate of IPD (%) [22] | 8.30 | 8.30 | 8.30 | 16.40 | 16.40 | 16.40 |
|
NBPP inpatient incidence |
16 | 84 | 261 | 131 | 403 | 632 |
|
NBPP outpatient incidence |
29 | 160 | 512 | 142 | 423 | 638 |
| Proportion of ST3 NBPP (%) [27] | 9.61 | 9.61 | 9.61 | 7.89 | 7.89 | 7.89 |
| Proportion of 22F + 33F NBPP (%) [27] | 10.92 | 10.92 | 10.92 | 11.50 | 11.50 | 11.50 |
| Proportion of V114 serotypes (%) [27] | 54.87 | 54.87 | 54.87 | 51.90 | 51.90 | 51.90 |
| Case fatality rate for inpatient NBPP (%) [26] | 3.81 | 3.81 | 3.81 | 9.77 | 9.77 | 9.77 |
†Data obtained from unpublished CDC ABCs serotype data (2017) shared with the authors
ABCs Active Bacterial Core surveillance, AR at risk, CDC Centers for Disease Control and Prevention, HR high risk, IPD invasive pneumococcal disease, LR low risk, NBPP non-bacteremic pneumococcal pneumonia, ST serotype, V114 15-valent pneumococcal conjugate vaccine
Age-specific hospitalization rates of all-cause community-acquired pneumonia (CAP) were taken from a population-based cohort study of hospitalized adults with CAP conducted by Ramirez et al. [23]. Rates of outpatient all-cause CAP were obtained from a retrospective analysis of pneumonia-related healthcare utilization in the USA conducted by Tong et al. [24]. The proportion of all-cause CAP attributable to NBPP was estimated at 11% [3]. Rates of inpatient and outpatient NBPP were then split into LR, AR, and HR using the relative risk estimates and weighted by the prevalence of the healthy (LR), AR, and HR populations obtained from Weycker et al. [25].
The CFR of NBPP for outpatients was assumed to be zero, and the hospitalization rates among patients were assumed to be the same across the three risk groups. The CFR of NBPP for inpatients was obtained from a retrospective cohort study conducted by Wuerth et al. [26]. Serotype distribution and proportions were estimated from Isturiz et al. [27].
Cost Inputs
Economic burden was estimated from a publicly funded healthcare payer perspective [28, 29]. Total direct medical costs per episode of IPD or NBPP were obtained by multiplying the number of hospitalizations by the cost per hospitalization for IPD or NBPP (Table 2). Costs were adjusted to 2019 US dollars and discounted at an annual rate of 3%.
Table 2.
Economic model parameters [25]
| Age category, years | Costs of IPD per episode (in 2019 USD) | Cost of NBPP per episode (in 2019 USD) | |
|---|---|---|---|
| Inpatient | Outpatient | ||
| 18–64 | $55,622 | $23,384 | $760 |
| 65–69 | $31,298 | $11,423 | $574 |
| 70–74 | $33,775 | $11,055 | $633 |
| 75–79 | $30,009 | $11,215 | $695 |
| 80–84 | $26,445 | $10,419 | $889 |
| 85–89 | $20,790 | $7681 | $882 |
| 90–100 | $20,368 | $8746 | $1342 |
IPD invasive pneumococcal disease, NBPP non-bacteremic pneumococcal pneumonia, USD US dollars
Source of cost data: Weycker et al. and MSD internal analysis using Humana claims data
Model Assumptions
The model makes a few assumptions: (1) The movement between risk categories as the person ages is not possible; (2) a patient may develop IPD or NBPP in 1 year; and (3) after PMS, patients would not develop another episode of IPD or NBPP, because such a situation was considered rare. Therefore, PMS is an absorbing state.
Model Outputs
Modeled outcomes included the number of IPD cases and deaths, the number of NBPP cases and deaths, and the overall total direct costs attributable to IPD and NBPP combined, and that were attributable to all V114 serotypes, serotypes 22F and 33F combined, and serotype 3 individually.
Scenario Analyses
Scenario analyses were conducted by varying the incidence and cost data by ± 20% and varying the discount rate to 0% and 5% to assess the lifetime total direct medical costs attributable to V114 serotypes in adults ≥ 19 years of age.
Results
IPD and NBPP Lifetime Cases by Serotype
For the approximately 250 million adults ≥ 19 years of age, the model estimated 415,229 cases of IPD that were attributable to V114 serotypes. Among these, 135,838 (32.7%) and 149,476 (36%) cases were caused by serotypes 22F + 33F and serotype 3, respectively (Table 3).
Table 3.
Lifetime IPD and NBPP cases attributable to all V114 serotypes, serotypes 22F + 33F, and serotype 3
| Age group | Risk cohorts | V114 serotypes | Serotypes 22F + 33F | Serotype 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IPD | NBPP | Total | IPD | NBPP | Total | IPD | NBPP | Total | ||
| 19–49 | HR | 30,974 | 702,334 | 733,308 | 9883 | 139,792 | 149,675 | 10,677 | 122,949 | 133,627 |
| AR | 54,803 | 1,409,522 | 1,464,325 | 17,487 | 280,550 | 298,037 | 18,892 | 246,748 | 265,641 | |
| LR | 142,751 | 3,081,986 | 3,224,737 | 45,550 | 613,435 | 658,985 | 49,210 | 539,527 | 588,738 | |
| 50–64 | HR | 26,443 | 654,787 | 681,230 | 8438 | 130,328 | 138,766 | 9116 | 4613 | 13,729 |
| AR | 40,941 | 1,123,466 | 1,164,408 | 13,064 | 223,613 | 236,677 | 14,114 | 8319 | 22,433 | |
| LR | 38,896 | 976,445 | 1,015,341 | 12,411 | 194,351 | 206,762 | 13,409 | 7442 | 20,851 | |
| 65–74 | HR | 16,896 | 443,924 | 460,820 | 6094 | 98,337 | 104,430 | 7155 | 67,532 | 74,688 |
| AR | 24,231 | 698,435 | 722,666 | 8739 | 154,715 | 163,454 | 10,262 | 106,250 | 116,512 | |
| LR | 10,760 | 307,890 | 318,649 | 3881 | 68,203 | 72,083 | 4557 | 46,838 | 51,395 | |
| 75+ | HR | 10,984 | 339,779 | 350,762 | 3961 | 75,267 | 79,228 | 4652 | 51,689 | 56,341 |
| AR | 13,738 | 449,944 | 463,681 | 4955 | 99,670 | 104,624 | 5818 | 68,448 | 74,266 | |
| LR | 3813 | 129,197 | 133,010 | 1375 | 28,619 | 29,994 | 1615 | 19,654 | 21,269 | |
| Total | 415,229 | 10,317,709 | 10,732,938 | 135,838 | 2,106,878 | 2,242,716 | 149,476 | 1,290,011 | 1,439,487 | |
Note: Numbers may not add up due to rounding
AR at risk, HR high risk, IPD invasive pneumococcal disease, LR low risk, NBPP non-bacteremic pneumococcal pneumonia, V114 15-valent pneumococcal conjugate vaccine
The number of cases of NBPP attributable to V114 serotypes was 10,317,709; 2,106,878 (20.4%) cases were associated with serotypes 22F + 33F and 1,290,011 (12.5%) cases were caused by serotype 3, respectively. The numbers of IPD and NBPP cases by age groups are provided in Table 3.
In older patients ≥ 65 years of age, the majority of IPD and NBPP cases attributable to V114 serotypes occurred in AR and HR patients.
IPD and NBPP Lifetime Mortality by Serotype
Of the estimated 57,192 deaths associated with V114 serotypes among patients with IPD, 18,811 (32.9%) and 20,780 (36.3%) deaths were due to serotypes 22F + 33F and serotype 3, respectively (Table 4).
Table 4.
Lifetime IPD and NBPP deaths attributable to all V114 serotypes, serotypes 22F + 33F, and serotype 3
| Age group | Risk cohorts | V114 serotypes | Serotypes 22F + 33F | Serotype 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IPD | NBPP | Total | IPD | NBPP | Total | IPD | NBPP | Total | ||
| 19–49 | HR | 3540 | 20,660 | 24,199 | 1129 | 4112 | 5242 | 1220 | 3617 | 4837 |
| AR | 6892 | 48,142 | 55,034 | 2199 | 9582 | 11,781 | 2376 | 8428 | 10,804 | |
| LR | 18,285 | 115,923 | 134,209 | 5835 | 23,073 | 28,908 | 6304 | 20,293 | 26,597 | |
| 50–64 | HR | 3570 | 26,352 | 29,922 | 1139 | 5245 | 6384 | 1231 | 4613 | 5844 |
| AR | 5815 | 47,522 | 53,336 | 1855 | 9459 | 11,314 | 2004 | 8319 | 10,324 | |
| LR | 5571 | 42,513 | 48,084 | 1778 | 8462 | 10,239 | 1920 | 7442 | 9363 | |
| 65–74 | HR | 2584 | 20,381 | 22,965 | 932 | 4515 | 5447 | 1094 | 3100 | 4195 |
| AR | 3821 | 32,155 | 35,976 | 1378 | 7123 | 8501 | 1618 | 4892 | 6510 | |
| LR | 1707 | 14,211 | 15,918 | 616 | 3148 | 3764 | 723 | 2162 | 2885 | |
| 75+ | HR | 2081 | 17,380 | 19,461 | 751 | 3850 | 4601 | 881 | 2644 | 3525 |
| AR | 2603 | 23,021 | 25,624 | 939 | 5099 | 6038 | 1102 | 3502 | 4604 | |
| LR | 723 | 6612 | 7335 | 261 | 1465 | 1725 | 306 | 1006 | 1312 | |
| Total | 57,192 | 414,871 | 472,063 | 18,811 | 85,132 | 103,944 | 20,780 | 70,018 | 90,798 | |
Note: Numbers may not add up due to rounding
AR at risk, HR high risk, IPD invasive pneumococcal disease, LR low risk, NBPP non-bacteremic pneumococcal pneumonia, V114 15-valent pneumococcal conjugate vaccine
The number of deaths attributable to V114 serotypes in patients diagnosed with NBPP was 414,871. In total, 85,132 (20.5%) and 70,018 (16.9%) deaths were attributable to serotypes 22F + 33F and serotype 3, respectively (Table 4).
In the 19–49 years of age and 50–64 years of age groups, more deaths were observed in the LR and AR groups. In older adults (≥ 65 years of age), higher numbers of deaths were observed in the AR and HR populations.
Economic Impact by Serotype
Total discounted lifetime costs (direct medical costs; US dollars) of IPD and NBPP attributable to all V114 serotypes were estimated to be $44.8 billion; serotypes 22F + 33F and serotype 3 accounted for $10.3 billion (22.9%) and $9.3 billion (20.7%) of total direct medical costs, respectively (Table 5). Generally, AR and HR patients in the ≥ 65 years of age groups were associated with a higher economic burden. In the 19–49 years of age and 50–64 years of age groups, the economic burden was higher for the LR and AR subgroups.
Table 5.
Lifetime discounted direct 2019 medical costs (USD, millions) for IPD and NBPP attributable to all V114 serotypes, serotypes 22F + 33F, and serotype 3
| Age group | Risk cohorts | V114 serotypes | Serotypes 22F + 33F | Serotype 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IPD | NBPP | Total | IPD | NBPP | Total | IPD | NBPP | Total | ||
| 19–49 | HR | $787.0 | $2783.3 | $3570.3 | $251.1 | $554.0 | $805.1 | $271.3 | $487.2 | $758.5 |
| AR | $1138.1 | $4565.3 | $5703.4 | $363.1 | $908.7 | $1271.8 | $392.3 | $799.2 | $1191.5 | |
| LR | $2846.4 | $8229.2 | $11,075.7 | $908.3 | $1637.9 | $2546.2 | $981.3 | $1440.6 | $2421.8 | |
| 50–64 | HR | $706.1 | $3203.2 | $3909.3 | $225.3 | $637.6 | $862.9 | $243.4 | $560.7 | $804.2 |
| AR | $951.9 | $4600.1 | $5552.1 | $303.8 | $915.6 | $1219.4 | $328.2 | $805.3 | $1133.5 | |
| LR | $889.0 | $3452.1 | $4341.2 | $283.7 | $687.1 | $970.8 | $306.5 | $604.3 | $910.8 | |
| 65–74 | HR | $350.7 | $1782.4 | $2133.1 | $126.5 | $394.8 | $521.3 | $148.5 | $271.2 | $419.7 |
| AR | $476.1 | $2633.9 | $3110.0 | $171.7 | $583.4 | $755.2 | $201.6 | $400.7 | $602.3 | |
| LR | $209.0 | $1098.2 | $1307.2 | $75.4 | $243.3 | $318.6 | $88.5 | $167.1 | $255.6 | |
| 75+ | HR | $207.7 | $1312.9 | $1520.6 | $74.9 | $290.8 | $365.7 | $87.9 | $199.7 | $287.7 |
| AR | $259.6 | $1738.2 | $1997.8 | $93.6 | $385.0 | $478.7 | $110.0 | $264.4 | $374.4 | |
| LR | $72.0 | $498.9 | $570.9 | $26.0 | $110.5 | $136.5 | $30.5 | $75.9 | $106.4 | |
| Total | $8893.7 | $35,897.8 | $44,791.6 | $2903.4 | $7348.8 | $10,252.2 | $3190.0 | $6076.3 | $9266.3 | |
Note: Numbers may not add up due to rounding
AR at risk, HR high risk, IPD invasive pneumococcal disease, LR low risk, NBPP non-bacteremic pneumococcal pneumonia, USD US dollars, V114 15-valent pneumococcal conjugate vaccine
Scenario Analysis
The scenario analysis indicated that lifetime total direct medical costs were sensitive to changes around all key parameters. Total costs were most affected by the discount rate, whereby total IPD and NBPP costs attributable to V114 serotypes increased by 46% and decreased by 18% for 0% and 5% discount rates, respectively (Table 6). When the incidence and total disease cost for IPD and NBPP were varied by ± 20%, total costs increased and decreased by 20% accordingly. Similar trends were observed for IPD and NBPP cases attributable to serotypes 22F + 33F and serotype 3.
Table 6.
Scenario analyses: lifetime total direct medical costs (in 2019 USD, millions) of IPD and NBPP attributable to V114 serotypes in adults
| Scenario | V114 | 22F + 33F | Serotype 3 |
|---|---|---|---|
| Base case | $44,791.6 | $10,252.2 | $9266.3 |
| Incidence + 20% | $53,728.6 | $12,297.7 | $11,115.2 |
| Incidence − 20% | $35,847.5 | $8205.0 | $7416.0 |
| Disease cost + 20% | $53,749.9 | $12,302.6 | $11,119.6 |
| Disease cost − 20% | $35,833.3 | $8201.7 | $7413.1 |
| Discount rate 0% | $65,539.3 | $15,001.1 | $13,558.6 |
| Discount rate 5% | $36,537.1 | $8362.8 | $7558.7 |
IPD invasive pneumococcal disease, NBPP non-bacteremic pneumococcal pneumonia, USD US dollars, V114 15-valent pneumococcal conjugate vaccine
Discussion
Our study used a modeling approach to estimate the lifetime burden of PD among adults in the USA. This modeling analysis demonstrated PD attributable to V114 serotypes as a major driver of healthcare utilization and costs in a hypothetical non-vaccinated cohort of US adults ≥ 19 years of age, followed until death. We estimated approximately 11 million lifetime cases of PD and 0.5 million deaths. A substantial proportion of these cases were caused by serotypes 3, 22F, and 33F. PCV13 serotype 3 and non-PCV13 serotypes 22F and 33F were responsible for 13.4% and 20.9% of PD cases, respectively. The number of cases associated with these three serotypes was disproportionate when compared with overall incidence due to all V114 serotypes. In another retrospective observational study conducted in the USA in 2017, 12.4–13.6% of IPD cases in adults were attributed to serotypes 22F and 33F; serotype 3 accounted for 11.6–15.5% of IPD cases [30]. A similar increase in cases attributed to serotype 22F after PCV13 introduction to the vaccination program was reported in Canada [31], Australia, and some European countries [32]. Several other studies have highlighted the persistence of some vaccine serotypes in adults after the introduction of PCVs, especially serotype 3 [11, 32, 33]. This evidence suggests a need for vaccines with greater effectiveness against vaccine serotypes that are persistent and continue to contribute significantly to disease burden, such as serotype 3, and that also extend coverage to include highly prevalent non-PCV13 serotypes causing substantial disease burden.
Zhang et al. previously reported a higher rate of IPD in US adults with HR or AR conditions than in healthy adults [34]. Moreover, costs of PD are substantially higher among persons with certain chronic and immunocompromising conditions [25]. Our findings continue to indicate that PD rates are substantially higher among older adults ≥ 65 years of age with AR or HR conditions when compared with healthy adults of the same age, with a similar trend seen for PD-related mortality and costs.
Our study also estimated a substantial economic burden of PD, accounting for approximately $45 billion in lifetime direct medical costs, with notable and disproportionate costs attributable to serotypes 3, 22F, and 33F. Huang et al. estimated $3.5 billion in direct medical costs associated with PD in the USA in 2004 [6]. Prevention of pneumococcal pneumonia with vaccines extending coverage to include serotypes 22F + 33F and increased effectiveness against serotype 3 might result in significant cost savings and reduce the burden of PD.
Limitations
Our study had a few limitations. First, our results were based on a modeling analysis that may not provide an accurate representation of the real future situation. Second, the analysis did not include direct non-medical costs borne by families or caregivers, such as transportation and lodging, or any indirect costs associated with productivity loss. Consequently, overall estimates of health and economic burden of IPD and NBPP associated with V114 serotypes were conservative. Although individuals aged through the model over their lifetime and their health risk changed substantially, the current model assumed the same risk for each age throughout their lifetime. This underestimated the PD-related cases, mortality, and costs associated with the risk groups over time.
Given that the treatment and management of IPD and NBPP depends on antibiotics, there is the potential for the development of resistance to specific antibiotics due to mutation over time. Currently, many bacteria, including some S. pneumoniae, have become resistant to one or more antibiotics [35], leading to treatment failure and extra treatment-associated costs. The current analysis did not include the associated health and economic impact of antimicrobial resistance. Finally, the current model did not include the cost of death attributable to PD, thereby underestimating the total cost presented in this study.
Strengths
Despite the limitations outlined above, the current analysis has several strengths. First, to our knowledge, this is the first study that focused on estimating the health and economic outcomes attributable to the 15 serotypes contained in V114. Second, given the importance of serotype-specific information for PDs, the model provided the relevant serotype-specific outcome information. The current analysis included all adults ≥ 19 years of age by risk category.
The value of next-generation adult pneumococcal vaccines should, therefore, not be limited to prevention of disease caused by serotypes not currently covered. This analysis demonstrates that to achieve full value in addressing the ongoing burden of PD, next-generation vaccines should account for both currently licensed vaccine serotypes as well as non-vaccine serotypes.
Conclusions
Using our Markov model, we estimated that, in the USA, V114 serotypes were associated with a substantial health and economic burden of PD among adults ≥ 19 years of age, the majority of which was attributable to serotypes 3, 22F, and 33F. The addition of V114 to the national vaccination program could reduce hospitalizations, costs, and deaths associated with PD in non-vaccinated adults ≥ 19 years of age in the USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The paper and Journal’s Rapid Service Fee was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Medical Writing, Editorial, and Other Assistance
Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments was provided by Suparna Abraham, PharmD, and editorial support was provided by Ian Norton, PhD, all of Scion, London, supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Kwame Owusu-Edusei, Arijita Deb, and Kelly D. Johnson conceptualized and determined the scope of the study. Kwame Owusu-Edusei performed the study analyses. Kwame Owusu-Edusei, Arijita Deb, and Kelly D. Johnson reviewed the inputs, results and the interpretation of the results. Kwame Owusu-Edusei, Arijita Deb, and Kelly D. Johnson critically reviewed and revised the manuscript for intellectual content. All the authors approved submission of this manuscript.
Disclosures
Kwame Owusu-Edusei and Kelly D. Johnson are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Arijita Deb was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA at the time of the development of the manuscript; currently, she is an employee of GSK. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Compliance with Ethics Guidelines
This article does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The original article was revised to an error in figure 1.
Change history
3/6/2022
A Correction to this paper has been published: 10.1007/s40121-022-00613-z
References
- 1.Centers for Disease Control and Prevention. The Pink Book: epidemiology and prevention of vaccine-preventable diseases. 14th ed. Chapter 17: pneumococcal disease. 2021. https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html. Accessed 23 Aug 2021.
- 2.Centers for Disease Control and Prevention. Surveillance and reporting. 2017. https://www.cdc.gov/pneumococcal/surveillance.html. Accessed 3 Apr 2019.
- 3.Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS ONE. 2013;8(4):e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelton SI, Bornheimer R, Doroff R, Shea KM, Sato R, Weycker D. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 immunization. Clin Infect Dis. 2019;68(11):1831–1838. doi: 10.1093/cid/ciy800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–989. doi: 10.1136/thoraxjnl-2015-206780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SS, Johnson KM, Ray GT, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–3412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) report: Streptococcus pneumoniae, 2018. 2018. https://www.cdc.gov/abcs/reports-findings/survreports/spneu18.html. Accessed 21 Jul 2021.
- 8.Ozawa S, Portnoy A, Getaneh H, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff (Millwood) 2016;35(11):2124–2132. doi: 10.1377/hlthaff.2016.0462. [DOI] [PubMed] [Google Scholar]
- 9.Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(46):1069–1075. doi: 10.15585/mmwr.mm6846a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu PJ, Hung MC, Srivastav A, et al. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill Summ. 2021;70(3):1–26. doi: 10.15585/mmwr.ss7003a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4):512e1–e10. doi: 10.1016/j.cmi.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Adam HJ, Golden AR, Karlowsky JA, et al. Analysis of multidrug resistance in the predominant Streptococcus pneumoniae serotypes in Canada: the SAVE study, 2011–15. J Antimicrob Chemother. 2018;73(suppl_7):vii12–vii9. doi: 10.1093/jac/dky158. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) Prescribing Information. 2021. https://www.fda.gov/media/150819/download. Accessed 21 Jul 2021.
- 15.Ermlich SJ, Andrews CP, Folkerth S, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults >/=50 years of age. Vaccine. 2018;36(45):6875–6882. doi: 10.1016/j.vaccine.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Dowell SF, Whitney CG, Wright C, Rose CE, Jr, Schuchat A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9(5):573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasbach EJ, Elbasha EH. Verification of decision-analytic models for health economic evaluations: an overview. Pharmacoeconomics. 2017;35(7):673–683. doi: 10.1007/s40273-017-0508-2. [DOI] [PubMed] [Google Scholar]
- 18.Shiri T, Khan K, Keaney K, Mukherjee G, McCarthy ND, Petrou S. Pneumococcal disease: a systematic review of health utilities, resource use, costs, and economic evaluations of interventions. Value Health. 2019;22(11):1329–1344. doi: 10.1016/j.jval.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 19.United States Census Bureau. Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2010 to July 1, 2019 2020. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html. Accessed 15 Apr 2021.
- 20.Arias E, Xu JQ. United States life tables, 2018. National Vital Statistics Reports. 2020. https://www.cdc.gov/nchs/data/nvsr/nvsr69/nvsr69-12-508.pdf. Accessed 12 Jul 2021. [PubMed]
- 21.Ahmed SS, Pondo T, Xing W, et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions—United States. Clin Infect Dis. 2020;70(12):2484–92. [DOI] [PubMed]
- 22.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) report: Streptococcus pneumoniae, 2017. 2017. https://www.cdc.gov/abcs/reports-findings/survreports/spneu17.html. Accessed 15 Apr 2020.
- 23.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 24.Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with pneumonia in the United States during 2008–2014. BMC Health Serv Res. 2018;18(1):715. doi: 10.1186/s12913-018-3529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16:182. doi: 10.1186/s12913-016-1432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuerth BA, Bonnewell JP, Wiemken TL, Arnold FW. Trends in pneumonia mortality rates and hospitalizations by organism, United States, 2002–2011(1) Emerg Infect Dis. 2016;22(9):1624–1627. doi: 10.3201/eid2209.150680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isturiz RE, Ramirez J, Self WH, et al. Pneumococcal epidemiology among us adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37(25):3352–3361. doi: 10.1016/j.vaccine.2019.04.087. [DOI] [PubMed] [Google Scholar]
- 28.Stoecker C, Kobayashi M, Matanock A, Cho BH, Pilishvili T. Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine. 2020;38(7):1770–1777. doi: 10.1016/j.vaccine.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 29.MSD. Internal analysis from 65+ BOD study. 2020. Data on File.
- 30.Grant LR, Suaya JA, Pugh S, Chilson E. Coverage of the 20-valent conjugate vaccine against invasive pneumococcal disease by age group in the United States, 2017. In: 12th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); 2020 June 20–24. Toronto, Canada (abstract number ID 237).
- 31.Demczuk WH, Martin I, Griffith A, et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Can J Microbiol. 2013;59(12):778–788. doi: 10.1139/cjm-2013-0614. [DOI] [PubMed] [Google Scholar]
- 32.Lochen A, Croucher NJ, Anderson RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. 2020;10(1):18977. doi: 10.1038/s41598-020-75691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Petigara T, Yang X. Clinical and economic burden of pneumococcal disease in US adults aged 19–64 years with chronic or immunocompromising diseases: an observational database study. BMC Infect Dis. 2018;18(1):436. doi: 10.1186/s12879-018-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Center for Disease Control and Prevention. Drug-resistant Streptococcus pneumoniae. 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/strep-pneumoniae-508.pdf. Accessed 5 Aug 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


