Figure 4.
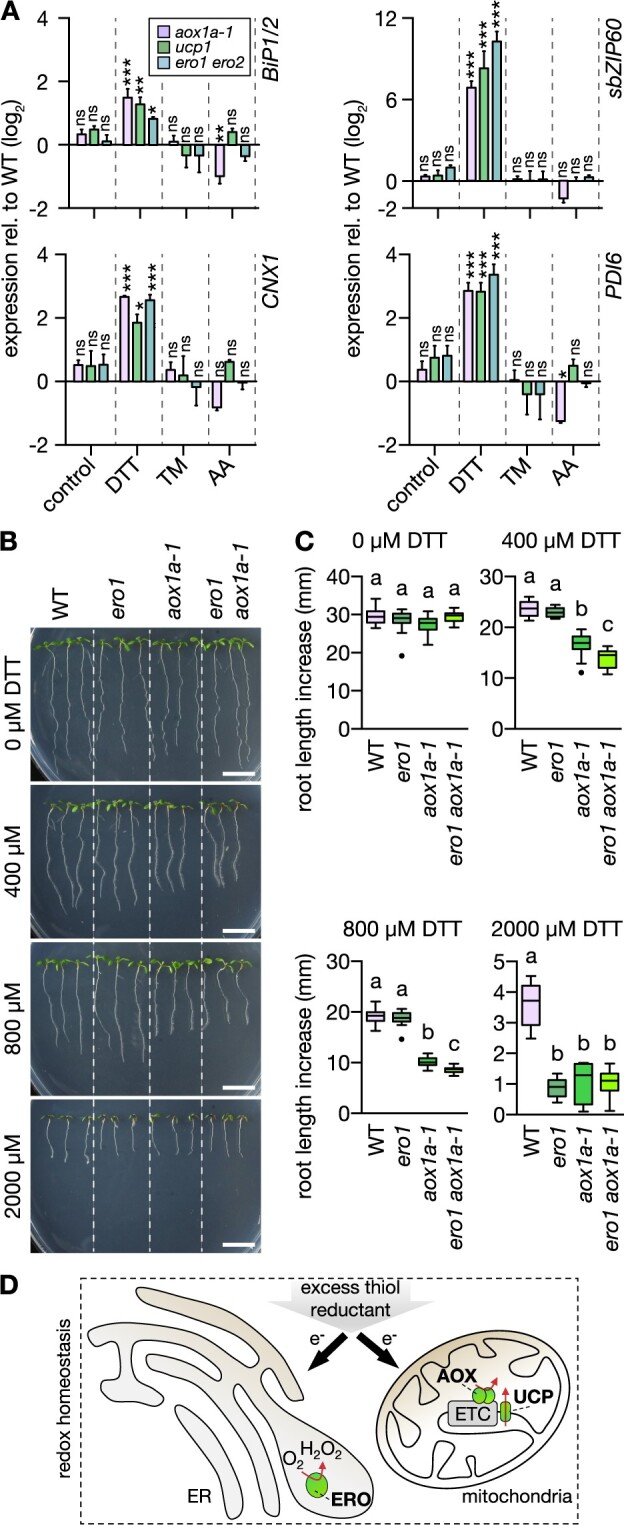
aox1a and ucp1 seedlings suffer from more severe ER stress at DTT exposure. A, ER stress marker transcript quantification by RT-qPCR from roots of 12-day-old seedlings submerged in treatment solutions without (control) or with 2-mM DTT or 4.3 µg mL−1 TM for 2.5 h. Lines: WT Col-0, aox1a-1, ucp1, ero1 ero2. Transcripts: BiP1/2, sbZIP60, CNX1, and PDI6. Expression in the mutants relative to WT (raw values in Supplemental Data Set 15); bars above and below black horizontal line indicate expression higher or lower relative to WT, respectively. Mean + sd. N = 3 plates with pooled roots from 18 to 20 seedlings each, except for DTT-treated WT and aox1a-1 (N = 2 plates). Differences were tested by two-way ANOVA with Dunnett’s multiple comparisons test (nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001). B, Primary root length increase of WT and transgenic seedlings (ero1, aox1a, and ero1 aox1a-1). Seedlings were grown vertically on half-strength MS agar plates for 4 days, then transferred to fresh plates supplemented with 0–2,000 µM DTT. Representative images of seedlings 4 days after transfer. Scale bars: 10 mm. C, Root length increase measured 4 days after transfer. 0 µM: N = 14–15, 400 µM: N = 15, 800 µM: N = 15, 2,000 µM: N = 15. Boxplot: first and third quartiles with median and Tukey’s whiskers. Differences were tested after log-transformation of data to establish normal distribution. Different letters indicate significant differences (one-way ANOVA with Bonferroni’s multiple comparisons test; P < 0.01). P-values: Supplemental Data Set 4. D, Rationale of how excess thiol reductant in the form of externally supplied DTT may be detoxified by both the ER through ERO activity and by the mitochondria through respiration.
