Learning objectives.
By reading this article, you should be able to:
-
•
Describe how bacterial toxins may bypass rate-limiting steps in systemic inflammation, triggering harmful levels of T-lymphocyte activation.
-
•
Know when to suspect that toxin release may be a factor in a patient's illness.
-
•
Explain the different strategies that may be used to reduce toxin concentrations.
Key points.
-
•
Shock and multiorgan failure that seems disproportionate to the inciting infection can be caused by bacterial toxins.
-
•
Exotoxins are responsible for several different disease states. Examples include toxic shock syndrome, streptococcal toxic shock syndrome, tetanus and botulism.
-
•
Exotoxins can cause local inflammation and tissue breakdown, helping bacterial spread.
-
•
Diagnosis of toxic shock syndrome can be difficult. Treatment should be initiated based on clinical suspicion, often triggered by clinical features, such as rashes.
-
•
Some antibiotics, such as clindamycin, act within bacterial ribosomes, reducing the production of exotoxins.
Infection is a common cause of morbidity and mortality. This is often a result of direct bacterial damage and the subsequent host inflammatory response. An often-overlooked additional contributing factor may be toxin release by certain specific organisms. This article discusses bacterial toxins and describes the common disease states they cause.
Toxins are generally categorised into endotoxins and exotoxins. Endotoxins are lipopolysaccharides (LPS) and cause Gram-negative sepsis. Exotoxins are peptides that are mostly secreted by Gram-positive bacteria. Some exotoxins act as superantigens and can trigger dysregulated host immune responses with widespread T-lymphocyte activation. This in turn may lead to life-threatening shock and multiorgan failure. This article discusses the pathophysiology of these superantigens, the clinical and therapeutic implications and their contrast with the more treatable effects of more familiar toxin-mediated specific diseases, many of which are largely preventable by vaccination. This may help clinicians better understand failures of clinical response to antimicrobial treatment.
Clinical scenario.
A 37-yr-old female with no known comorbidities presented with high-grade fever, shortness of breath and gastroenteritis.
Examination findings: pulse 140 beats min−1, BP 70/40 mmHg, ventilatory frequency 28 bpm and SpO2 88% whilst breathing room air.
The patient had a generalised erythematous rash with cold extremities. There was no organomegaly, neck rigidity or focal neurological deficit. A small burn was noted on the distal aspect of her right thumb with serosanguinous discharge.
A CT pulmonary angiogram revealed bilateral ground glass changes in the lungs with no evidence of pulmonary embolism, and right-sided axillary lymphadenopathy with no evidence of abscess or necrosis. CT scan of her abdomen and pelvis did not identify any source of sepsis. Transthoracic echocardiography demonstrated features of cardiogenic shock but no valvular vegetations.
Treatment with broad-spectrum antibiotics was started. Blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA), and a wound swab from the right thumb also grew MSSA.
Her clinical condition deteriorated despite early sterilisation of blood cultures with appropriate antibiotics and commencement of multiple organ support, including mechanical ventilation and inotropic drugs. Given the clinical picture of toxicity out of proportion to local findings and a generalised erythematous rash, leading to unexpected deterioration with multiorgan failure, toxic shock syndrome was suspected. Sadly, she died 7 days after hospital admission.
Bacterial toxins
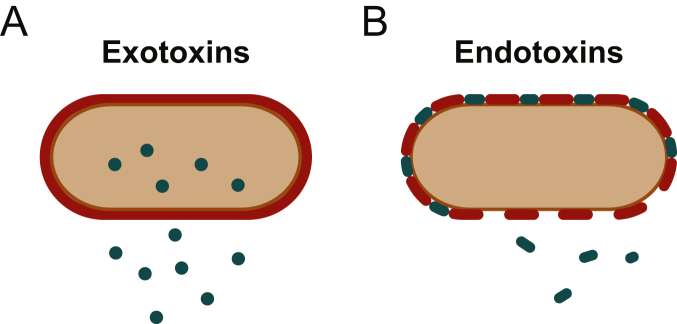
Bacteria can cause host damage via the release of toxins; they are divided into endotoxins and exotoxins (Fig. 1). Endotoxin is an LPS that forms part of the bacterial cell wall. It is ineffective at eliciting durable antibody responses and does not readily denature with heat. Whilst endotoxins are released at a constant low rate from live bacteria, much higher concentrations are released during bacterial cell lysis.
Fig 1.
(A) Bacterium secreting exotoxin produced inside the cell. (B) Bacterium releasing endotoxin from its cell wall.
Exotoxins are highly antigenic proteins. They are secreted at a constant low rate from inside bacteria or are released during bacterial cell lysis. Exotoxins can elicit potent antibody responses (underpinning vaccine development) and can also interact with receptors or other biological mechanisms to produce identifiable syndromes. The various forms of Clostridia are prime examples of bacteria that produce exotoxins. Some Gram-negative bacteria, such as Pseudomonas aeruginosa, produce exotoxins as do most Gram-positive bacteria. Exotoxins have been categorised into subtypes based on differing general properties and consequences (Table 1).
Table 1.
Features of endotoxins and exotoxins.
| Endotoxins |
Exotoxins |
|||
|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | ||
| Examples of typical diseases | Gram-negative bacteria causing sepsis | Gram-positive cocci causing toxic shock syndromes | Gram-positive anaerobes causing clostridial myonecrosis, Clostridium difficile colitis | Gram-positive anaerobes causing tetanus and botulism |
| Chemistry | Lipopolysaccharide | Polypeptide | Polypeptide | Polypeptide |
| Stimulates adaptive immune response | No | Yes | Yes | Yes |
| Mechanism of action | Interacts with TLR-4 and CD14, activating CD4 cells, causing the release of TNF-α and IL-1 | Superantigen activates large numbers of T4-lymphocytes causing exaggerated immune response | Enzymatic or pore-forming activity, affecting cell membranes and cell matrix | A (active) and B (binding) toxins and others that can also affect cell function |
| Vaccines | No | No | No | Yes; toxoids used as vaccines |
| Heat stability | Stable at 100°C for >1 h | Destroyed by heat >60°C (except staphylococcal enterotoxin) | Destroyed by heat >60°C | Destroyed by heat >60°C |
| Typical bacteria | Escherichia coli, Pseudomonas and Klebsiella | Staphylococcus aureus and Streptococcus pyogenes | Clostridium perfringens and Clostridium difficile | Clostridium tetani and Clostridium botulinum |
Endotoxin
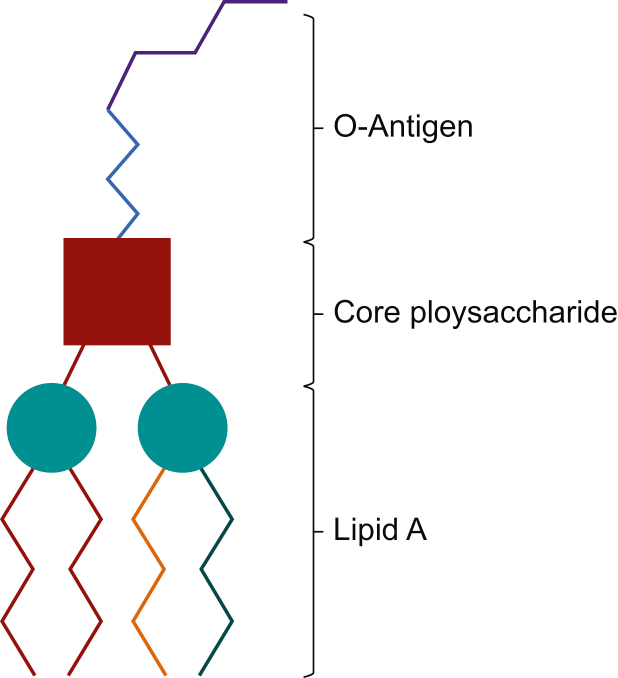
Lipopolysaccharide is shown in Fig. 2 as complexes of lipid A, core oligosaccharide and O-antigen (O-polysaccharide). Lipid A is composed of a disaccharide molecule, myristic acid and other fatty acids specific to bacterial species. Despite being highly toxic, it does not stimulate an adaptive immune response, leaving patients at risk of repeat episodes of endotoxin-mediated shock. The core oligosaccharide is characteristic of the bacterial genus and is composed of oligosaccharide, phosphate and amino acids.
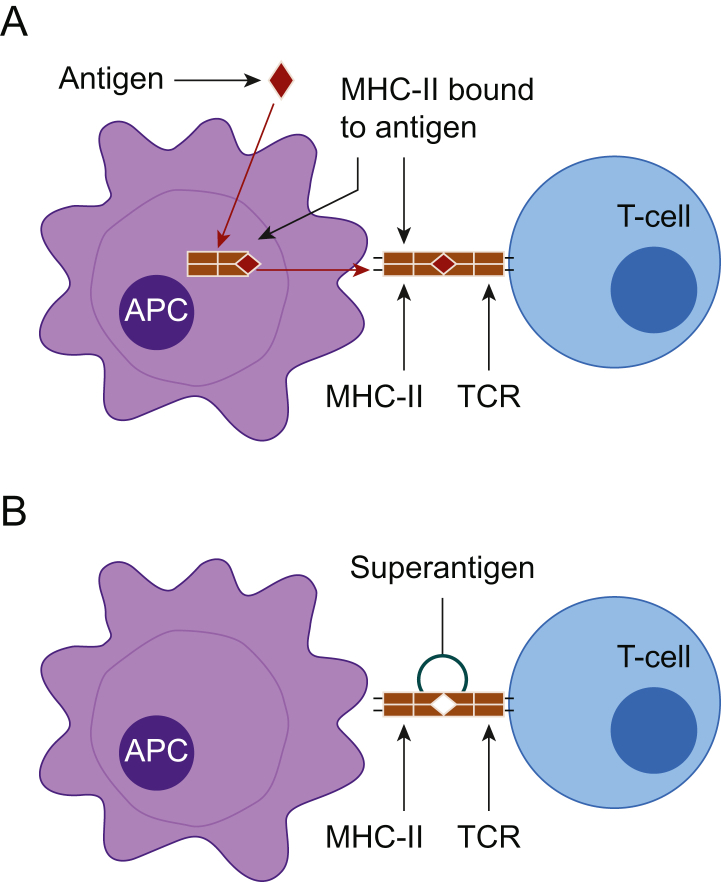
Fig 3.
(A) Specific antigen-presenting cells (APCs) engulf and process antigenic material intracellularly and express the antigen on their cell surface bound to major histocompatibility complex (MHC)-II. These bound antigens are only recognised by T4 lymphocytes having a T-cell receptor (TCR) with a corresponding shape. (B) Superantigen attaching directly onto MHCII on the APC and TCR on T4 lymphocytes activating the T4 lymphocyte non-specifically.
Lipopolysaccharides and LPS-binding protein form a complex with CD14 and Toll-like receptor (TLR)-4 on the surface of CD4 cells, activating intracellular nuclear factor (NF)-κβ. A function of NF-κβ is regulation of cytokine gene transcription, including tumour necrosis factor (TNF)-α and interleukin (IL)-1. High systemic LPS concentrations trigger dysregulated cytokine release through this mechanism and along with complement activation may lead to septic shock and multiorgan dysfunction.1,2
Type 1 exotoxins: the concept of the superantigen
Normally, a complex process of antigen processing mediates the activation of T4 lymphocytes, serving to limit and coordinate the immune response. Specific antigen-presenting cells (APCs) engulf and process antigenic material inside the cell and express the antigen on their cell surface bound to major histocompatibility complex (MHC)-II. These bound antigens are only recognised by T4 lymphocytes having a T-cell receptor (TCR) with a corresponding shape (Fig. 3). This specificity is an essential and rate-limiting step in the activation of T4 lymphocytes in most infections. The feedback mechanisms regulating this process typically ensure that only a small fraction, much less than 1% of T4 lymphocytes, is activated. However, certain superantigens can bypass this requirement for intracellular processing. They attach more directly to the MHC-II and TCR activating T4 lymphocytes non-specifically (Fig. 3). This step, which forgoes the rate-limiting intracellular processing step, is very rapid and can lead to more direct activation of large numbers of T4 lymphocytes, with as many as 40% of the circulating total being activated. Activated T4 lymphocytes release interferon-γ, which mediates the release of extremely high amounts of IL-1, IL-6 and TNF from APCs. In turn, this may lead to rapid progression of shock and multiorgan dysfunction. Examples of Type I exotoxins include toxic shock syndrome (TSS) toxin, released by Staphylococcus aureus causing TSS, and pyrogenic exotoxin released by Streptococcus pyogenes, causing streptococcal toxic shock syndrome (STSS).
Fig 2.
Lipopolysaccharide molecule made up of O-antigen, core polysaccharide and lipid A.
Type 2 exotoxins: mediators that damage host cell membranes
These exotoxins are usually either pore-forming cytotoxins or phospholipase enzymes with lytic activity to intracellular structures. They cause destruction of host cell membranes and extracellular matrix by eliciting an inflammatory response either directly or through the release of damage-associated molecular patterns. Damage-associated molecular patterns are signal molecules released by damaged or infected host cells, which can directly interact with TLR, leading to inflammatory cytokine release. This may serve to facilitate spread of infection along tissue planes or into previously healthy tissue.
Type 3 exotoxins: ‘A–B toxins’
Classically, these exotoxins consist of two parts: an ‘A’ (active) component and a ‘B’ (binding) component. The A components are enzymatically active and, through inactivation of intracellular signalling pathways, interfere with host cell functions. Generally, this involves the process of ADP-ribosylation, catalysing the removal of ADP-ribosyl from NAD+ co-enzyme and attaching it to host cell proteins, affecting their function. The binding components, which are receptors on the host cell membrane, determine which cells are affected. Once bound, exotoxins are either endocytosed or the A component passes directly into the host cytosol. Some bacteria are equipped with a Type III secretion system, which uses a needle-like structure to introduce exotoxins directly into the host cell cytoplasm. Type 3 exotoxins often produce recognisable and familiar illnesses, such as tetanus and botulism. These exotoxins can be used to make toxoids, which are safe but remain antigenic, and as such are the bases for various vaccines.
Toxin-mediated diseases
Gram-negative septic shock
Patients with endotoxic shock present with non-specific manifestations of sepsis. Examples include fever, increased ventilatory frequency, tachycardia, hypotension and signs of organ hypoperfusion (such as altered mental status and oliguria). Neutrophilia, increased C-reactive protein and procalcitonin concentrations and biochemical evidence of organ failure are common. Positive blood cultures may later confirm diagnosis, but treatment should be commenced empirically based on clinical suspicion. In managing endotoxic shock, rapid recognition, antibiotics, resuscitation with fluids and early vasopressor and other organ support remain the mainstays of therapy.3
Specific modalities directly targeted at endotoxin pathways have been studied, but to date none have shown clinical benefit. Treatment with anti-endotoxin human monoclonal IgM antibodies (centoxin) was not associated with any significant improvement in mortality in the Centocor: HA-1A Efficacy in Septic Shock (CHESS) trial group RCT.4 Therapies aimed at removal of endotoxin from serum by haemofiltration were developed. However, despite promise shown by preliminary studies, the Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock (EUPHRATES) study, showed no improvement in mortality.5,6 A Cochrane review concluded that there was insufficient evidence to recommend i.v. immunoglobulin as standard of care in endotoxin-mediated sepsis.7
TSS and STSS
Toxic shock syndrome can occur as a result of wound infections, osteomyelitis, mastitis, sinusitis and burns, particularly in immunocompromised patients. It can also be seen because of retained or unhygienic tampon use. After an influenza-like prodromal illness, fever, severe myalgias and gastrointestinal upset can often occur with superficial soft tissue infection. Suspected cases should be examined for foreign bodies. This examination should include vaginal examination for retained tampons in females, although non-menstrual TSS is far commoner than menstrual TSS. Staphylococcus aureus also produces exfoliative exotoxins (proteases), which cause destruction of the desmosome layer and subsequent scalded skin syndrome.8
Invasive group A streptococcal infection is a risk factor for STSS, most commonly in the form of necrotising soft tissue infection. Streptococcal toxic shock syndrome is typically associated with a history of early onset of severe pain disproportionate to external signs, followed by erythema, ecchymoses and skin sloughing, and it can progress to necrotising fasciitis. In addition to producing a superantigen, S. pyogenes releases cytotoxic enzymes, such as streptokinase and DNases. These enzymes may help spread infection through infarcted planes.8
Patients with TSS and STSS often rapidly develop multiorgan failure, more so than in other infections caused by these organisms. Toxic shock syndrome is defined by an erythematous macular rash, whilst this rash is only present in 10% of cases of STSS. Desquamation, particularly affecting palms and soles, usually occurs 1–2 weeks after onset. Its presence is generally only used for retrospective confirmation rather than being of any diagnostic use. Clinical case definitions have been detailed for both syndromes.8
The diagnosis of TSS and STSS is based on clinical criteria. Positive blood cultures are rare in staphylococcal TSS (<5%) compared with STSS (>60%). Isolation of Gram-negative organisms on laboratory cultures makes TSS less likely. Other laboratory findings will be consistent with septic shock and multiorgan dysfunction. As there is no specific laboratory test available to confirm diagnosis, treatment must be commenced based on clinical suspicion and suggestive history.8
If exotoxin activity is suspected, standard treatment for suspected septic shock should be commenced.3 Commonly used empirical regimes, such as piperacillin–tazobactam plus vancomycin, will cover both Gram-positive and Gram-negative organisms. However, additional cover with clindamycin is often advised to decrease exotoxin production. In the UK, methicillin-resistant S. aureus is isolated from 5.6% of proven S. aureus bacteraemias, and clindamycin-resistant Group A Streptococcus is isolated from 9% of Group A streptococcal bacteraemias.9,10 Linezolid should be used for exotoxin reduction if resistant species are suspected.8 The need for source control of infection through surgical debridement in STSS or removal of foreign body, such as a tampon in TSS, must also be urgently considered.8 I.V. immunoglobulin treatment improves outcomes in STSS. I.V. immunoglobulin does not have such a well-recognised role in the treatment of TSS, but it should be considered for this condition as well.11 Whilst a detailed discussion of the full range of Type II exotoxins produced by Staphylococcus and Streptococcus species is beyond the scope of this article, Panton–Valentine leucocidin (PVL) produced by S. aureus deserves special mention. This pore-forming exotoxin causes leucocyte destruction, commonly presenting as recurrent skin infections or abscesses. Rarely, a more fulminant form presents as haemorrhagic necrotising pneumonia. Methicillin-resistant S. aureus cover should be provided because of its association with PVL. The presence of PVL can make treatment of infections especially difficult, increasing the requirement for surgical intervention. Despite this, a meta-analysis did not report increased mortality rates in patients with PVL-positive bacteraemia (10%) vs patients with PVL-negative bacteraemia (16%). Panton–Valentine leucocidin testing is recommended for patients with recurrent or community-acquired invasive staphylococcal infections.12
Clostridial myonecrosis
Clostridium perfringens is a ubiquitous, facultatively anaerobic, spore-forming Gram-positive bacillus. Infection is usually via a penetrating injury and is associated with impaired cardiovascular function. This produces an anaerobic environment optimal for further bacterial growth. Clostridium perfringens produces a variety of exotoxins, which lead to gas gangrene as the devitalised tissue area expands. Virulence depends on the exotoxins produced. Alpha toxin, a lecithinase that causes local tissue breakdown, with platelet aggregation, thrombosis and reduced perfusion, extends the area of devitalised tissue. Once absorbed systemically, alpha toxin causes haemolysis, cardiac suppression and subsequent toxic shock. Theta toxin (perfringolysin O) also attacks vascular tissues and shows leucocidal activity and so causes a dysregulated cytokine mediated host response manifesting as toxic shock. Other exotoxins break down connective tissue, allowing bacterial spread, increasing cell wall permeability and causing local oedema. Necrotising toxins, designated beta, iota and nu toxins, are also produced. Clostridial myonecrosis (gas gangrene) presents with severe pain disproportionate to injury at the site of trauma. Skin may initially seem minimally inflamed, soon becoming pale and discoloured and then developing discharging bullae characterised by a musty smell. Affected patients often deteriorate rapidly and develop septic shock with multiorgan failure.13
Clostridium difficile
Clostridium difficile is an anaerobic, spore-forming Gram-positive bacillus, widely distributed in the intestinal tract and in the environment. Infection occurs when spores, which are very resilient structures, are transmitted through the faecal–oral route. With disruption of the normal gut flora, spores germinate and C. difficile colonises the gut. Clostridium difficile produces Type II exotoxins, designated A and B, which inactivate GTPase in colonic epithelial cells. Both have enterotoxic and cytotoxic activities. Through disruption of the cytoskeleton, hypersecretion of fluid, neutrophil adhesion and local inflammation, they disrupt gut barrier integrity and functionality.
Clinical features can range from asymptomatic carriage to septic shock and toxic megacolon. Clostridium difficile should be suspected in patients who develop abdominal pain and watery diarrhoea 3–9 days after commencement of antibiotics. Older or frail patients are at particular risk. Stools will be foul smelling, greenish in colour and positive on faecal occult blood testing. Severe cases result in pseudomembranous colitis. Systemic effects are attributable to exotoxin-mediated cytokine release, and patients can present with severe shock, abdominal distension, toxic megacolon and bowel perforation.14
Tetanus
Clostridium tetani is an obligate anaerobic, spore-forming Gram-positive bacillus. Tetanus spores enter the body through contaminated trauma. After an incubation period of 3–21 days, bacteria divide and release tetanus toxin (tetanospasmin). Tetanospasmin travels via retrograde axonal transport from the peripheries to the spinal cord, where it prevents inhibitory neurotransmitter release. Generalised tetanus may ensue, or it may be localised. Muscle rigidity, spasms and autonomic dysfunction occur. Generalised tonic rigidity is usually the first symptom with trismus dysphagia, stiffness of the neck or truncal muscles noted. Periodic tetanic spasms can occur. Sometimes spontaneous, they are more commonly provoked by physical or emotional stimuli. Apnoea may result from laryngospasm and thoracic wall rigidity. Respiratory failure is the leading cause of death in tetanus. Autonomic dysregulation is present in severe cases, with tachycardia, hypertension, dysrhythmias and even cardiac arrest. Less severe features include ileus, increased salivation, increased bronchial secretions and urinary retention. Although now a rare disease, tetanus should be recognised by intensive care clinicians.15,16
Botulism
Clostridium botulinum is a ubiquitous, obligate anaerobe, spore-forming Gram-positive bacillus. When an appropriate anaerobic environment prevails, the spores germinate and grow into exotoxin-producing bacteria. Botulinum toxin enters presynaptic nerve cells of voluntary muscles and of the autonomic nervous system. In the nerve terminal, they prevent acetylcholine release by inhibiting fusion of its vesicles to the cytosolic membrane. Botulinum toxin is one of the most lethal toxins known, with a dose of 1 ng kg−1 being potentially fatal. It leads to the descending symmetrical flaccid paralysis characteristic of botulism.17,18
Diagnostic techniques
Most exotoxin-associated disease states have characteristic clinical features. Hence, treatment should be generally initiated based on clinical suspicion rather than awaiting laboratory confirmation. Investigations exist, which can support diagnosis. Culture and microscopy techniques, either of blood, wound or site of infection (e.g. nasopharyngeal swab for Bordetella pertussis) are useful. Some exotoxin-producing bacteria are ubiquitous in nature and have non-toxigenic varieties and long incubation periods, making cultures unhelpful (e.g. tetanus wound culture). Polymerase chain reaction tests may allow distinctions to be drawn when non-toxigenic varieties of bacteria exist. Bioassays may detect exotoxins in samples from host tissues. Most involve enzyme immunoassays, such as enzyme-linked immunosorbent assay. Historically, samples containing exotoxins were injected directly into living animals to check for symptoms (e.g. mouse bioassay for botulism).17 Testing the serum for exotoxin immunoglobulin can be used, where low levels of antitoxin immunoglobulin suggest disease susceptibility (e.g. in tetanus).19
Adjunctive antitoxin therapies
To reduce exotoxin-producing bacteria in the body, surgical debridement of infected wounds and treatment with targeted antibiotic therapy should be initiated as early as possible. The choice of antibiotic may be based on local guidelines and later amended once sensitivity information from cultures is available. A variety of treatment strategies are outlined in Table 2.
Table 2.
Treatment strategies. ∗Exotoxin reducing.
| Disease | Treatment strategies |
|---|---|
| Toxic shock syndromes | Broad-spectrum antibiotics Clindamycin or linezolid∗ i.v. immunoglobulin Surgical debridement |
| Clostridial myonecrosis | Broad-spectrum antibiotics Surgical debridement Clindamycin∗ |
| Clostridium difficile | Oral vancomycin Oral fidaxomicin Oral/i.v. metronidazole Surgery for toxic megacolon Faecal transplantation in recurrent disease |
| Tetanus | Metronidazole Antitoxin antibodies Surgical debridement Vaccination |
| Botulism | Antitoxin antibodies Wound debridement Vaccination (high risk only) |
Clindamycin is a bacteriostatic antibiotic that acts by binding to the 50S ribosomal subunit of bacteria. Clindamycin reduces exotoxin production in Gram-positive bacteria and is recommended as adjuvant therapy to help reduce exotoxin load in the body.20 Alternatively, linezolid, with a similar mechanism of action, has also been shown to reduce exotoxin production. Linezolid should be considered when there is intolerance of clindamycin or suspected resistance to clindamycin.20
Because exotoxins are proteins, toxoids can be produced by denaturing the exotoxin with heat or formaldehyde. This denatured toxoid is immunogenic without being toxigenic. Tetanus and diphtheria toxoid vaccines are developed in this way. Alternative methods for vaccine production include whole cell dead bacterium and purified antigen, both of which have been used for B. pertussis vaccines. Generally, coadministration of tetanus, diphtheria and pertussis vaccine is part of the childhood schedule. Five doses are currently recommended by the WHO to confer long-term protection. Guidelines for the management of tetanus prone wounds in unvaccinated patients are available at https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals.19
Summary
Bacterial infection can lead to host damage in three general ways: direct tissue damage, autoimmune mechanisms and by the release of specific bacterial toxins. Lipopolysaccharide, an endotoxin released from the cell wall of Gram-negative bacteria, plays a significant role in the pathophysiology of Gram-negative sepsis. Some bacteria secrete peptide exotoxins, which can be further classified based on their mechanism of action. Type 1 exotoxins can have superantigen activity, leading to massive T4 lymphocyte activation and dysregulated cytokine release, leading to TSS or STSS, which can be difficult to diagnose. Type 2 exotoxins can lead to cell membrane or cell matrix destruction through their enzymatic or pore-forming activity, helping bacteria to spread or causing massive tissue destruction. Type 3 exotoxins can interact with cellular mechanisms causing pathognomonic disease states.
An understanding of the important role that these toxins have will improve early recognition and early effective treatment of these conditions. In turn, this will improve patients' outcomes. Close communication between the intensive care, infectious diseases and microbiology teams is required to manage these complex patients.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
John Robert Sheehan BSc FCAI FJFICMI is a specialty registrar in anaesthesiology and intensive care medicine at Cork University Hospital.
Corinna Sadlier FRCPI PhD is a consultant in infectious diseases at Cork University Hospital, where she is chair of the antimicrobial stewardship committee. She is a member of the national immunisation advisor committee, and her areas of research include vaccine-preventable infections and immunological responses to vaccines in immunocompromised patients.
Brian O'Brien M Med Sc (Physiol) FCARCSI FJFICMI FCICM (ANZ) FFSEMI is a consultant in anaesthesiology and intensive care medicine and chair of the department at Cork University Hospital. He is also a member of the council of the College of Anaesthesiologists of Ireland, where he has served as chairman of the training committee.
Matrix codes: 1A01, 2B06, 3C00
References
- 1.Alexander C., Rietschel E.T. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 2.Brown M.A., Jones W.K. Department of pharmacology and cell biophysics, 231 albert sabin way ML0575, university of cincinnati, cincinnati, OH 45267-0575. Sepsis. 2004;1:1201–1217. [Google Scholar]
- 3.Seymour C.W., Liu V.X., Iwashyna T.J., et al. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCloskey R.V., Straube R.C., Sanders C., Smith S.M., Smith C.R. Treatment of septic shock with human monoclonal antibody HA-1A: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1994;121:1–5. doi: 10.7326/0003-4819-121-1-199407010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Chang T., Tu Y.K., Lee C.T., et al. Effects of polymyxin B hemoperfusion on mortality in patients with severe sepsis and septic shock: a systemic review, meta-analysis update, and disease severity subgroup meta-analysis. Crit Care Med. 2017;45:e858–e864. doi: 10.1097/CCM.0000000000002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellinger R.P., Bagshaw S.M., Antonelli M., et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320:1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alejandria M.M., Lansang M.A.D., Dans L.F., Mantaring J.B. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. 2013;2013 doi: 10.1002/14651858.CD001090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappin E., Ferguson A.J. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9:281–290. doi: 10.1016/S1473-3099(09)70066-0. [DOI] [PubMed] [Google Scholar]
- 9.Public Health England . PHE; 2020. Annual epidemiological commentary: Gram-negative bacteraemia, MRSA bacteraemia, MSSA bacteraemia and C. difficile infection data, up to and including financial year April 2020 to March 2021; pp. 1–96. GOV-9331. [Google Scholar]
- 10.Public Health England Laboratory surveillance of pyogenic and non-pyogenic streptococcal bacteraemia in England: 2019. Health Prot Rep. 2020;14:1–19. [Google Scholar]
- 11.Parks T., Wilson C., Curtis N., Norrby-Teglund A., Sriskandan S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:1434–1436. doi: 10.1093/cid/ciy401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shallcross L.J., Fragaszy E., Johnson A.M., Hayward A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:43–54. doi: 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao P., Annamaraju P. StatPearls; 2020. Clostridium perfringens.https://www.ncbi.nlm.nih.gov/books/NBK559049/ Available from. [PubMed] [Google Scholar]
- 14.Czepiel J., Dróżdż M., Pituch H., et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen L.M., Thwaites C.L. Tetanus. Lancet. 2019;393:1657–1668. doi: 10.1016/S0140-6736(18)33131-3. [DOI] [PubMed] [Google Scholar]
- 16.Taylor A.M. Tetanus. Contin Educ Anaesth Crit Care Pain. 2006;6:101–104. [Google Scholar]
- 17.Horowitz B.Z. Botulinum toxin. Crit Care Clin. 2005;21:825–839. doi: 10.1016/j.ccc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Wenham T., Cohen A. Botulism. Contin Educ Anaesth Crit Care Pain. 2008;8:21–25. [Google Scholar]
- 19.Amirthalingam G., Godbole G., Chand M., et al. 2019. Tetanus: guidance on the management of suspected tetanus cases and on the assessment and management of tetanus-prone wounds.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/820628/Tetanus_information_for_health_professionals_2019.pdf Available from. [Google Scholar]
- 20.Coyle E.A., Cha R., Rybak M.J. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin A release. Antimicrob Agents Chemother. 2003;47:1752–1755. doi: 10.1128/AAC.47.5.1752-1755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]