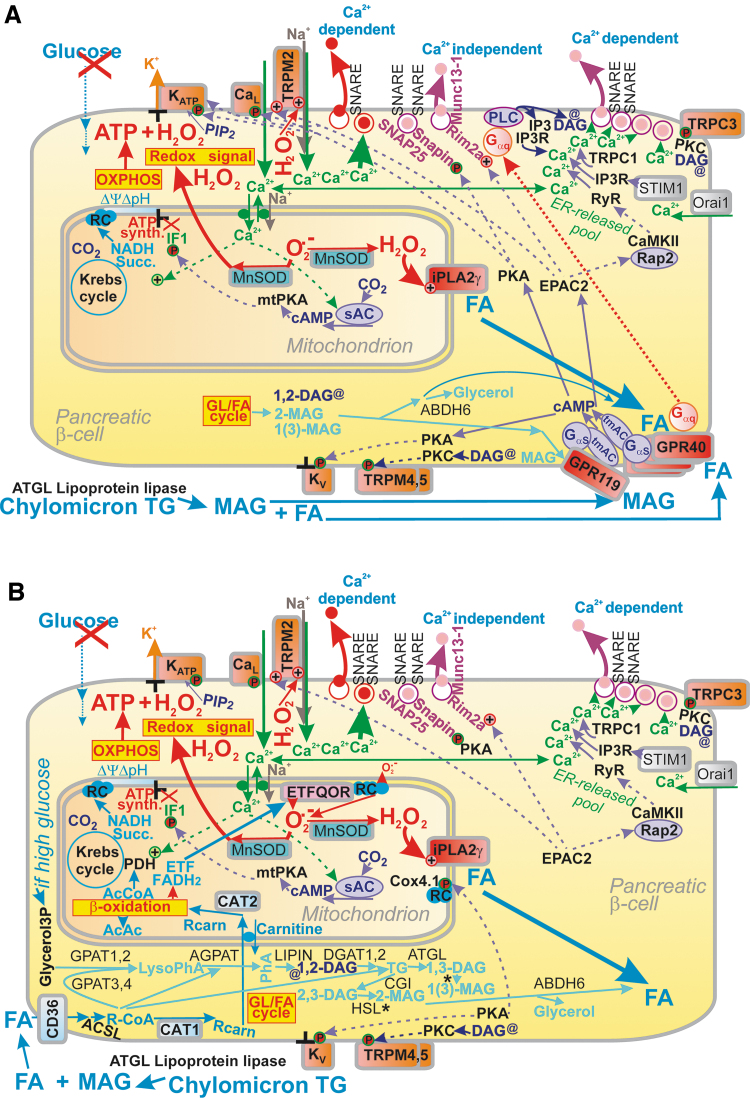
FIG. 10.
Receptor and metabolic pathways determining FASIS. (A) Receptor component of stimulation is emphasized. (B) Metabolic component is emphasized. When present in islets capillaries, for example, in incoming chylomicrons, FAs are cleaved by lipoprotein lipase (ATGL being the most specific β-cell isoform) and may either (A) act via receptor pathways to stimulate the GPR40 metabotropic receptor (94), in parallel with the second product of the cleavage, MAG, which signals via GPR119. Alternatively, (B) the metabolic pathway begins by FA import into the cell by the CD36 transporter and by ACSL conversion to acyl-CoAs. As for (A), the activation of both receptors leads to CaV-mediated action potential spikes and concomitant pulsatile insulin secretion. GPR40 acts via the Gαq/11, thus activating PLC, which leads to IP3 and DAG release (for DAG downstream pathway, see @). IP3 activates additional Ca2+ efflux from the ER via the IP3R, which is initiated either by the preceding CaV opening (95) or by PLC-TRPC-induced Ca2+ efflux from the ER (276). The most prominent pathway downstream of DAG involves the PKC-mediated phosphorylation of TRPM4 and TRPM5 to activate them. As a result, together with TRPM2, activated by Ca2+ and H2O2, these channels strengthen the necessary shift to −50 mV depolarization at the 100% closed KATP ensemble. The KATP closure is ensured by the metabolic component of FASIS (B). The two components are mutually interrelated since the canonical GPR119 signaling and the biased GPR40 signaling leads to the cAMP-mediated activation of the PKA and EPAC2 pathways (65, 187, 287). PKA phosphorylates the CaVβ2 subunit to activate it, phosphorylates KATP (see legend of Fig. 2) and inhibits Kv channels, which prolongs the already more intensive Ca2+ influx (179). Snapin, which allows IGV docking to the plasma membrane, is also PKA-phosphorylated, enabling initiation of the snapin SNARE complex with a lipid-anchored protein, the SNAP-25 (236). The EPAC2 pathway is based on its guanine nucleotide exchange activity. This induces further TRPM2 activation (285), regulates KATP (121) plus priming of the interaction of Rim2a with Munc13-1, required for the syntaxin 1 interaction of IGV [which activates IGV exocytosis (282)] and, finally, the activation of RyR- mediated Ca2+ efflux from the ER (120). FAs imported by CD36 are converted to acyl-CoAs by AcylCoA-synthetase (ACSL), whereas CAT1 converts acyl-CoAs to acyl-carnitines (207). The carnitine carrier (SLC25A20) imports acylcarnitines into the matrix, exchanging them for carnitine. The matrix CAT2 converts acyl carnitines to acyl-CoAs, which is followed by FA β-oxidation (see also Fig. 8). As described in the Mechanisms of Insulin Secretion section, all the benefits of activation also occur for mitochondrial metabolism, that is, activations upon GSIS and its receptor-mediated amplification (cf. Fig. 4). Also, similar redox signaling due to the increased superoxide formation upon FA β-oxidation occurs during the metabolic branch of FASIS, as with BCKA-stimulated insulin secretion (cf. Fig. 9). Elevated ATP from OXPHOS fortified by FA β-oxidation and elevated cytosolic H2O2 due to the increased H2O2-release from the matrix close the KATP channel (possibly also TRPM2), as they do upon GSIS. Overactivation of GPR40: pathways (A, B) are also interconnected because of the intramitochondrial redox signaling [elevated matrix superoxide/H2O2 due to FA β-oxidation (Fig. 11) directly activates mitochondrial phospholipase iPLA2γ/PNPLA8 (98, 103, 105)]. The phospholipase iPLA2γ cleaves both saturated and unsaturated FAs from the phospholipids of mitochondrial membranes. The cleaved free FAs diffuse up to the plasma membrane, where they activate GPR40 (103). FASIS in iPLA2γ-knockout mice or its isolated islets yields ∼30% insulin in the first fast phase of insulin secretion compared with wt mice (Holendová et al., unpublished data). This supports the existence of such an acute mechanism in vivo. Overactivation of GPR119: FASIS in the presence of high [glucose] (which by itself would stimulate GSIS) also involves the so-called glycerol/FA cycle combining simultaneous lipogenesis and lipolysis, as suggested by Prentki et al. (197). Enzymes involved in this cycle are described in the legend of Figure 8. An important intermediate of the glycerol/FA cycle is 1,2-DAG, which initiates PKC signaling (and TRPM4,5 activation) and activates Munc13-1 to facilitate IGV exocytosis. Moreover, created MAGs (* indicates its diffusion toward GPR119) can diffuse to the plasma membrane and overactivate the GPR119 receptor there. ABDH6, alpha/beta-hydrolase domain containing 6, monoacylglycerol lipase; CaMKII, Ca2+/calmodulin-dependent protein kinase II; FASIS, fatty acid-stimulated insulin secretion; IP3, inositol-1,4,5-triphosphate; IP3R, inositol-1,4,5-triphosphate receptor; iPLA2γ, Ca2+-independent phospholipase A2 isoform γ; Orai1, calcium release-activated calcium modulator 1; PLC, phospholipase C; Rap2, Ras-related protein 2; RC, respiratory chain; SNAP-25, synaptosomal nerve-associated protein 25; SNARE, soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor; tmAC, transmembrane adenylyl cyclase; TRPC, transient receptor potential canonical.