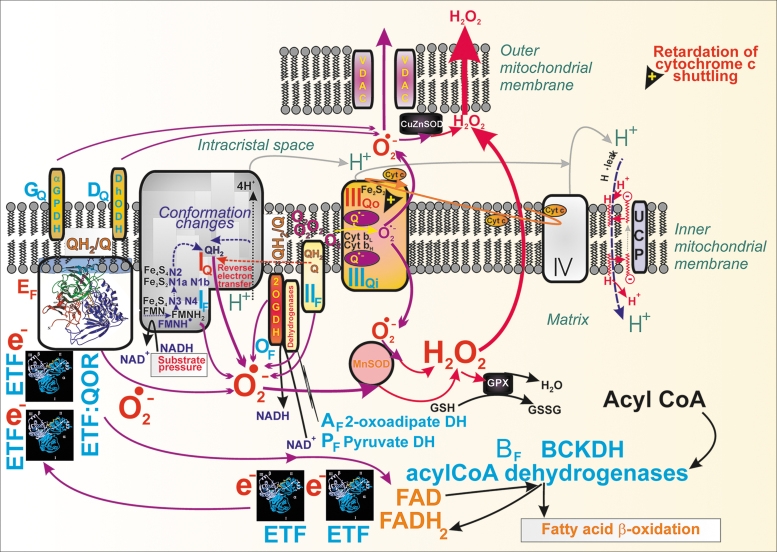
FIG. 11.
Superoxide formation due to FA β-oxidation or oxidation of BCKAs. An overview of locations for mitochondrial superoxide sources (blue capitalized fonts), termed according to the nomenclature introduced by Brand (25) is shown with those which increase superoxide formation upon FA β-oxidation or the β-like oxidation of BCKAs emphasized (in red). The electron-transfer flavoprotein:ubiquinone oxidoreductase (ETFQOR) is a common key electron transfer link between the initial dehydrogenases of these reactions and the respiratory chain complexes III and IV (29, 268). ETFQOR accepts electrons sequentially from two ETFs as single-electron carriers, while converting ubiquinone (Q) from its IMM pool to QH2. The electron leak from flavin to the oxygen leads to a radical pair formation and subsequent superoxide formation within the ETF:QOR itself (site EF). Moreover, the requirement of ETF:QOR to react with Q effectively outcompetes Q as the Complex I substrate, resulting in relative electron transfer retardation over the whole respiratory chain, hence superoxide is formed at its sites IQ and IIIQo. Finally, due to the increasing acetyl-CoA entry (propionyl-CoA entry for KIV; through methylmalonyl and succinyl-CoA) and NADH entry into the Krebs cycle, the excessive formation of superoxide at site IF may also contribute. After the conversion of superoxide to H2O2 by the matrix MnSOD and the intermembrane space CuZnSOD, the ongoing H2O2 efflux from mitochondria can be regarded as redox signaling. As in other types of mitochondria, there are in total six sites acting at the ∼280 mV redox potential of the NADH/NAD+ isopotential pool (index F, flavin) and five sites acting at the ∼20 mV redox potential of the QH2/Q isopotential pool (index Q) (25). Of these, only the superoxide sources with more intensive production at higher ΔΨm are attenuated by uncoupling proteins (103). This also involves the reverse electron transfer to the Complex I site IQ. In turn, superoxide formation at site IF increases with the increasing NADH/NAD+ ratio (“substrate pressure”). When cytochrome c shuttling (orange elliptic arrow) is retarded, then the Complex III site IIIQo provides major superoxide formation, which cannot be attenuated by uncoupling. IMM, inner mitochondrial membrane.