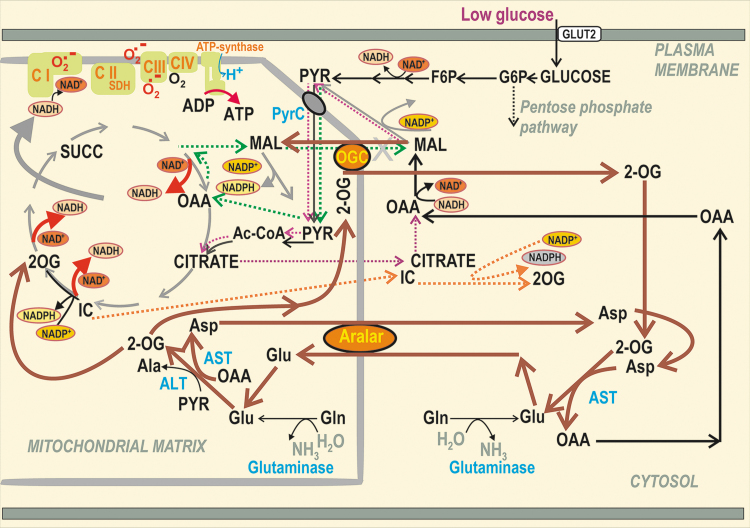
FIG. 7.
MAS is plausible when pyruvate-based redox shuttles are not operating. The MAS (brown) could participate in metabolic fluxes in pancreatic β-cells at low nonstimulating [glucose], when the pyruvate-based redox shuttles do not provide the opposite malate fluxes for the 2OGC. Moreover, the MAS transfers redox equivalents of NADH into the mitochondrial matrix; however, matrix NADH was found to decrease upon GSIS and relies on at least one of the two aspartate–glutamate antiporters, that is, SLC25A12/AGC1/aralar (18, 217) and SLC25A13/AGC2 (193) (data not shown). The key enzymes are alanine aminotransferases (cytosolic ALT1 and mitochondrial ALT2; also termed glutamate pyruvate transaminases, GPT1 and GPT2). Within MAS, ALT2 catalyzes the conversion of pyruvate plus l-glutamate to 2OG and l-alanine, whereas ALT1 (omitted for simplicity) would catalyze the reaction in the reverse mode. Analogously, there are aspartate aminotransferases, cytosolic AST1, and mitochondrial AST2 (also termed glutamate oxaloacetate transaminases, GOT1 and GOT2). In MAS, AST2 converts oxaloacetate plus l-glutamate to 2OG and l-aspartate, whereas AST1 should catalyze the opposite reaction to complete the cycle. Due to the reverse character of the aminotransferase reaction, its direction depends on the glutamate metabolism. AGC1, aspartate–glutamate antiporter SLC25A12 (Aralar); ALT, alanine aminotransferase; Aralar, aspartate–glutamate antiporter SLC25A12 (AGC1); AST, aspartate aminotransferase (aka glutamate oxaloacetate transaminase, GOT); GPT, glutamate pyruvate transaminase; MAS, malate/aspartate shuttle.