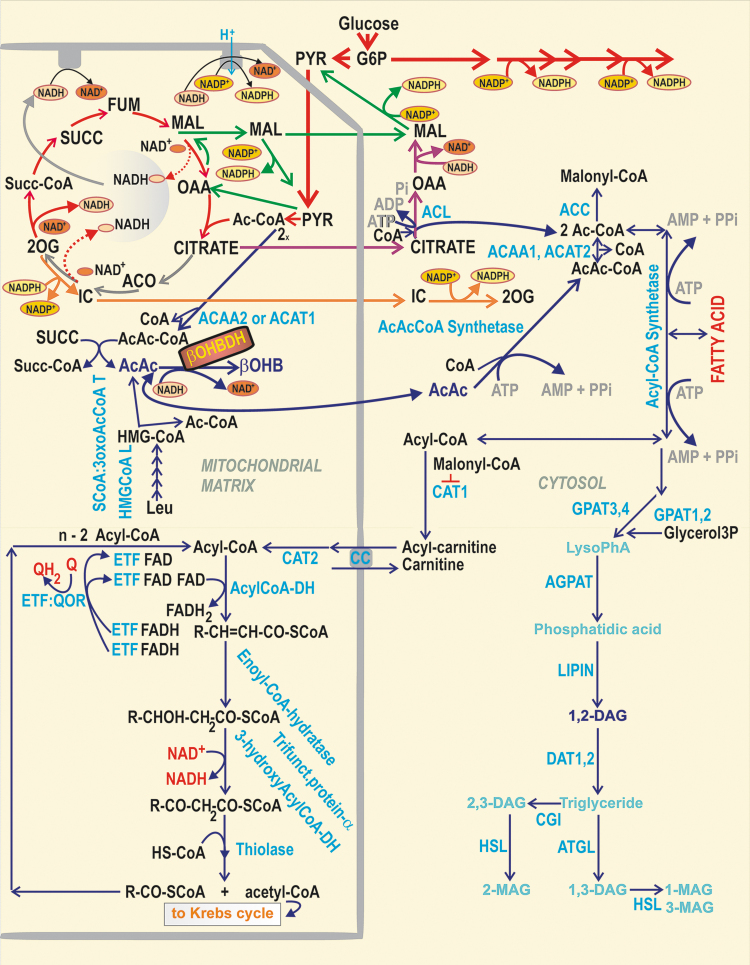
FIG. 8.
β-OHB formation and FA metabolism in pancreatic β-cells. The scheme describes three selected metabolic branches: (i) β-OHB formation and its relationship to leucine metabolism; (ii) FA β-oxidation; and (iii) the cytosolic glycerol/FA cycle. As for (i), at high [glucose], succinate is interconverted with AcAcCoA to S-CoA and acetoacetate by SCoA:3oxoAcCoAT (52). As part of leucine metabolism during a series of oxidative reactions (“β-like oxidation”) resembling FA β-oxidation, HMG-CoA is split by HMGCoAL into acetyl-CoA and acetoacetate. Besides being converted by β-OHBDH, acetoacetate can escape to the cytosol. Distinct enzyme isoforms convert two molecules of acetyl-CoA into CoA and AcAcCoA in the mitochondrial matrix. The latter are ACAT1 and ACAA2, whereas in the cytosol, there are ACAT2 and ACAA1. Cytosolic acetyl-CoA was suggested to facilitate the acetylation of proteins, which might speculatively enhance GSIS (189, 190). (ii) FA β-oxidation: FA is imported via CD36 into β-cells, where AcylCoA-synthetase (ACSL), localized externally to the ER membrane and OMM, converts FAs to acyl-CoAs, whereas the cytosolic CAT1 (synonymous for carnitine palmitoyltransferase, CPT1) converts acyl-CoAs to acylcarnitines (207). The carnitine carrier (SLC25A20) provides the import of acylcarnitines into the matrix, exchanging them for carnitine. The matrix CAT2/CPT2 converts acyl carnitines to acyl-CoAs. The following chain of reactions, termed FA β-oxidation, shortens the FA-acyl chain by two carbons, involving acyl-CoA dehydrogenases, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and β-thiolase. The product of a single cycle is just acyl-CoA shortened by two carbons plus acetyl-CoA. The FA β-oxidation is regulated via the inhibition of CAT1/CPT1 by malonyl-CoA, formed by ACC from acetyl-CoA. (iii) Cytosolic glycerol/FA cycle (197): elevated glucose is converted to glycerol3P, which is esterified by acyl-CoAs by GPATs (bound externally to ER and OMM) to LysoPhA. The latter is further esterified by AGPAT (bound to ER) to PhA. At the ER surface or lipid droplets, lipins transform PhA to 1,2-DAG, initiating PKC signaling and activating Munc13-1. DAG is also acylated there to TG, by diacylglycerol O-acyltransferase-1 and -2 (DGATs). Simultaneously, the lipolytic branch is provided by the cytosolic ATGL, hydrolyzing TG to DAG, upon the facilitation of perilipin (data not shown) and CGI-58 protein (CGI) on the lipid droplet surface. DAG is hydrolyzed to MAG by HSL, again facilitated by perilipin. The created MAGs can overactivate the GPR119 receptor (Fig. 10). The glycerol/FA cycle is completed by the hydrolysis of MAG to glycerol and FAs by the plasma membrane-associated ABHD6 (data not shown), whereas glycerol is exported from β-cells. β-OHB, β-hydroxybutyrate; β-OHBDH, β-hydroxybutyrate dehydrogenase; ABHD6, alpha/beta-hydrolase domain containing 6, monoacylglycerol lipase; ACAA, acetyl-CoA acyltransferase; AcAcCoA, acetoacetyl-CoA; ACAT, acetyl-CoA acetyltransferase; ACC, acetyl-CoA carboxylase; ACSL, long-chain acyl-CoA synthetase; AGPAT, 1-acylglycerol-3-phosphate acyltransferase; ATGL, adipose triglyceride lipase; CAT, carnitine acyltransferase; CGI, comparative gene identification 58, ATGL co-activator (aka ABDH5); CPT, carnitine palmitoyltransferase; DAG, diacylglycerol; DAT, DGAT, diacylglycerol O-acyltransferase; FA, fatty acid; glycerol3P, glycerol-3-phosphate; GPAT1,2, glycerol-3-phosphate acyltransferase 1,2; GPAT3,4, glycerol-3-phosphate acyltransferase 3,4 (1-acylglycerol-3-phosphate O-acyltransferase); GPR, G-protein-coupled receptor; HMG-CoA, hydroxymethyl-glutaryl-CoA; HMGCoAL, hydroxymethyl-glutaryl-CoA lyase; HSL, hormone-sensitive lipase; LysoPhA, lysophosphatidic acid; MAG, monoacylglycerol; OMM, outer mitochondrial membrane; PhA, phosphatidic acid; PKC, protein kinase C; SCoA:3oxoAcCoAT, succinyl-CoA:3-ketoacid-CoA transferase; Succ-CoA, S-CoA, i.e. succinyl-CoA; TG, triglyceride.