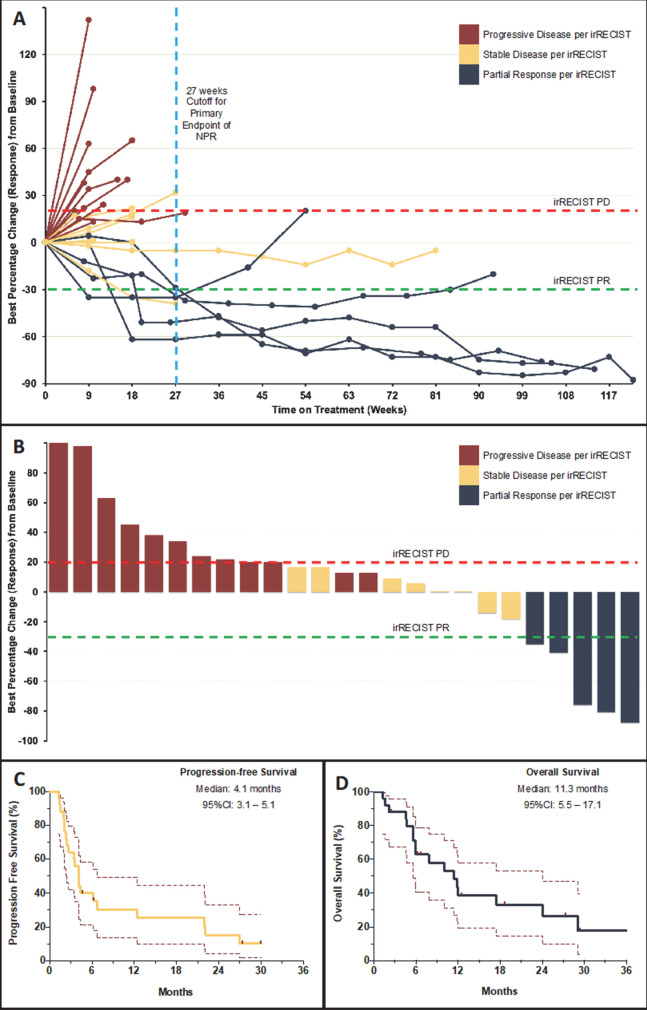
Figure 1.
Tumor response and survival outcomes on pembrolizumab in patients with cancer of unknown primary. (A) (Spider plot) shows the change in sum of target lesion diameters over time in 23 evaluable patients who were treated on the current study and underwent at least one radiological restaging evaluation (two patients had clinical progression prior to first restaging and are reported as default 20% increase). Two patients had unequivocal progression of non-target lesions and were considered as cases with progressive disease (PD). (B) (Waterfall-plot) shows the maximum per cent change from baseline as measured by immune-related Response Evaluation Criteria in Solid Tumors (irRECIST). Partial response (PR) was defined by ≥30% decrease in tumor burden and PD was defined by ≥20% increase in tumor burden, confirmed on a consecutive scan at least 4 weeks apart. (C and D) (KapIan-Meier curves) show progression-free survival and overall survival of patients on study at the time of data cut-off measured from treatment initiation to disease progression/death and death, respectively. Data from patients without an event were censored at date of last follow-up (marks). NPR, non-progression rate.