Abstract
Background
To examine the influence of mouth breathing on maxillofacial and airway development in children and adolescents with different cervical vertebral maturation stages.
Methods
Lateral cephalometric radiograph of a total of 120 children and adolescents, 64 girls and 56 boys (7–15 years old), diagnosed with mouth breathing were examined. Maxillofacial hard tissue, soft tissue and airway measurements were obtained using both manual and digital techniques. Independent samples t-test was performed to compare the difference between the measured indexes and the standard values.
Results
As for maxillofacial hard tissue, SNB (CS1–CS5), GoGn (CS1–CS5), ArGoNa (CS1–CS5), ArGo (CS1–CS2) and SNA (CS1–CS2) in mouth breathing children and adolescents were below the standard values (P < 0.05). NGoMe (CS1–CS5), SN-MP (CS1–CS4), SN-PP (CS1–CS4), PP-MP (CS1–CS3) and SN-GoGn (CS1–CS2) in mouth breathing children and adolescents were above the standard values (P < 0.05). As for maxillofacial soft tissue measurements, H angle (CS1–CS5), lower lip length (CS1–CS5), upper lip protrusion (CS1–CS5), upper lip length (CS1–CS4), lower lip protrusion (CS1–CS3), surface Angle (CS2–CS3) and nasolabial angle (CS2) in mouth breathing children and adolescents were above the standard values with statistically significance (P < 0.05). As for airway measurements, PAS (CS1, CS2, CS5) in mouth breathing children and adolescents was above the standard value with statistical significance (P < 0.05).
Conclusions
Mouth breathing had a real effect on maxillofacial and airway development, which differed among mouth breathing children and adolescents with different cervical vertebral maturation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-022-02234-x.
Keywords: Cervical vertebral maturation, Maxillofacial development, Airway development, Mouth breathing
Background
Chronic rhinitis, hypertrophy of the adenoid, palatine tonsils and deviated nasal septum are the frequent cause of upper respiratory obstruction, which forces children to breathe through their mouths [1]. Researches showed that mouth breathing incidence in children was 17–50% [2, 3]. Abnormal breathing patterns of mouth breathing leads to posteroinferior rotation of the mandible, inducing a prolonged imbalance of oropharynx muscle activity. Cranial and maxillofacial muscles produce a series of adaptive alterations, affecting the jaw's growth and development and leading to malocclusion [4, 5].
The craniofacial characteristics such as anterior overbite, deep overjet, poor lip seal, mandibular retrusion, and airway stenosis tend to worsen with the dentofacial growth of children with mouth breathing [6]. Stahl et al. investigated the relationship between cervical bone maturity and mandibular growth to infer that craniofacial growth in subjects with untreated Class II malocclusion had significantly smaller increases in mandibular length at the growth spurt and during the overall observation period [7]. Facial profile changes, the diagnosis of jaw bone disharmony, the most suitable intervention or treatment time, and the stability of the curative effect depend heavily on the growth characteristics and growth spurt of the maxilla and mandible. Therefore, understanding maxillofacial development and morphological characteristics in different stages is of great significance to the treatment planning, the control of tissue reconstruction, and the long-term prognosis. Luca [8] et al. took a cephalometric comparison about skeletal and dental features in ninety-eight children aged 7–12 years old who were split into two groups: mouth breathing secondary to nasal septum deviation and nasal breathing controls, finding that mouth breathing children displayed an increase of palatal height and overjet and upper and lower anterior facial height, a significantly retrognathic position of the maxilla and mandible, and the narrow of maxillary intermolar width. In addition, it was proposed that most mouth breathing children presented a class II skeletal malocclusion and cross-bite occurred more frequently than in the nasal breathing children. This is consistent with the traditional view that the facial type of mouth breathing children is mostly manifested as maxillary protrusion, mandibular retraction.
Helena [9], Isabel [10], Maria [11–13], Sara [14] et al., studied the influence of mouth breathing caused by upper respiratory tract obstruction, such as allergic rhinitis, nasal obstruction, adenoid/tonsil hypertrophy on maxillofacial development in children. Compared with the nasal breathing children, mouth breathing children showed the statistical insignificance of SN-PP and the increase of PP-MP, SN-MP, SN-OP, which reflected the minor secondary effect on maxilla, the occlusal plane steepened and the posteroinferior rotation of the mandible. The values of SPAS, PAS and other airway indicators in the mouth breathing group were decreased, indicating that airway stenosis was caused by mouth breathing in the process of posterior rotation of mandible.
The influence of mouth breathing on the maxillofacial development is still unsettled. The research results on the effects of mouth breathing on the anterior lower height of the face and the position of the maxilla [10, 15–17]. Contrary to previous studies [1, 18], Bernardo [19] et al. proposed that the ANB Angle of mouth breathing children was significantly larger than that of nasal breathing controls in both primary dentition and mixed dentition, while the SNB Angle decreased. Doron [20] et al. proposed that retrusive mandible in mouth breathing children was mainly manifested by deepened overjet and SNB Angle, which was inconsistent with Bernardo's results.
Compared with chronological age, skeletal age can more accurately reflect individual growth and maturity [21–23]. Maturational stages refer to specific developmental events identified on hand-wrist or cervical x-rays that relate directly to the progression of maturation during childhood and adolescence. Each progressive stage represents an increasing percentage of total facial skeletal growth completed. Although cervical vertebrae x-rays do not allow for such definitive evaluation as hand-wrist x-rays, the cervical vertebra maturity assessment system has a unique advantage that cephalometric radiographs are routine for orthodontic analysis and treatment planning avoiding additional X-ray exposure [24]. Hassel and Farman [25], Garcia-Fernandez et al. [26] have reported that cervical vertebral maturation analysis was comparable to hand-wrist analysis for assessing skeletal maturity, and it had high reliability and validity. And the variation of the cervical vertebra ossification center is more obvious to observe in the development period due to its fewer amount [27].
Due to the ongoing controversy and the lack of cervical vertebral maturation method in the effects of mouth breathing on maxillofacial and airway development, in this cross-sectional study, we made the statistical comparisons for cephalometric measurements to explore the craniofacial and airway growth changes in children and adolescents with mouth breathing, as defined by the cervical vertebral maturation method. Our study has three null hypotheses. The first null hypothesis is’mouth breathing affects the maxillofacial hard tissue development throughout all the growth and development period (for the cervical vertebrae maturation [CVM] method)’. The second null hypothesis is ‘mouth breathing affects the maxillofacial soft tissue development throughout all the growth and development period (for the cervical vertebrae maturation [CVM] method)’. The third null hypothesis is’mouth breathing affects the airway development throughout all the growth and development period (for the cervical vertebrae maturation [CVM] method)’.
Methods
Study design and setting
This cross-sectional study was conducted with the approval of the ethical committee of the Stomatological Hospital of Chongqing Medical University (ID: CQHS-REC-2020(LSNO.49)) in compliance with the Helsinki Deceleration and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Additional file 1). All the information was collected with informed consent from the guardians of the children and adolescents.
Study participants
According to the sample content calculation formula N = K*Q/P(K = 100,Q = 1-P when the allowed error is 20%,P is the expected incidence rate, and the incidence rate of adenoid hypertrophy is 49.7%), a total of 120 mouth breathing children and adolescents were retrospectively examined and included in this study during the period from December 2018 to September 2019. The age limit for participating in the study was 7–15 years of age. Combined with the results of the guardian's inquiry and clinical examination, the patient who fulfill the following two conditions could be classified as a mouth breath child or adolescent [10]: they had the habit of breathing through their mouth at rest or during sleep. At the same time, the child or adolescent was in a resting position with a double-sided mirror placed under the nostril observed for the presence of fogging or water vapor in the mouth side, or with a cotton wool placed under the nostril observed fluttering. Children and adolescents with the nasal inflammatory lesions or space-occupying lesions, history of other persistent oral habits, history of adenoidectomy or tonsillectomy, maxillofacial surgery history or history of orthodontic treatment were excluded from the analysis.
Variables and data measurement
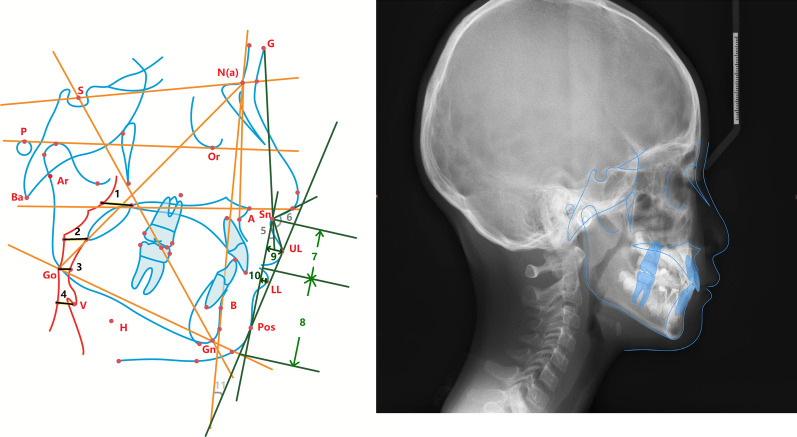
All patients in the current study were subjected to X-ray imaging in the intercuspal position. Cephalograms were routinely obtained with an X-ray diagnosis system (Kodak 9000; Kodak, Rochester, NY, USA) at a voltage of 62 kV, current of 8 mA and distance of median sagittal plane to the X-ray source of 154.5 cm. The digitized X-ray cephalograms were uploaded into the Dolphin 3D software (version 11.0; Dolphin Imaging, Chatsworth, CA). The detailed craniofacial hard tissue landmarks and measurement items that were established are shown in Fig. 1 and Table 1. 120 Subjects were then divided into six stages CS1-CS6 according to the method of cervical vertebral maturation assessment was described in Reilly’s study [28]. All Cephalometric measurements were measured twice in three months interval by two trained examiners using Dolphin 3D software, and the intra-class correlation coefficient was applied to analyze the internal reliability of observers. The mean of the two measured values was used for the final statistical analysis.
Fig. 1.
Cephalometric reference planes. Cephalometric index. 1 = PNS-UPW; 2 = U-MPW; 3 = PAS; 4 = V-LPW; 5 = surface Angle; 6 = nasolabial Angle; 7 = length of upper lip; 8 = length of lower lip; 9 = upper lip protrusion;10 = lower lip protrusion;11 = H Angle
Table 1.
Indicator definitions and standard values were included
| Item | Definition | Diagnostic value | Normal value |
|---|---|---|---|
| Craniofacial measurements | |||
| SNA | Angle formed by the sella-nasion line and line N-point A | Anteroposterior position of the maxilla in relation to the skull base | 82° |
| SNB | Angle formed by the sella-nasion line and line N-point B | Anteroposterior position of the mandible in relation to the skull base | 80.9° |
| ANB | Differences between the SNA and SNB angles | Relation between maxilla and mandible | 3° |
| Y | Anteroinferior angle between Y axis and FH plane | Protrusion of mental region, as well as the growth direction | 67° |
| FH-MP | Angle formed by the intersection of the FH plane and MP plane | Mandibular inclination | 25.9° |
| SN-MP | Angle formed by the intersection of the SN plane and MP plane | Mandibular inclination | 33° |
| PP-MP | Angle formed by the intersection of the PP plane and MP plane | Mandibular inclination | 25° |
| SN-PP | Angle formed by the sella-nasion line and palatal plane | The degree of the maxilla inclination in relation to the anterior cranial base | 8° |
| SN-GoGn | Angle formed by the sella-nasion line and mandibular plane | Inclination of the mandibular plane in relation to the skull base | 33° |
| GoGn | Linear distance between the gnathion and gonion | The length of mandibular body | 75.4° |
| ArGo | Linear distance between the articulare and gonion | The length of mandibular ramus | 39.3° |
| ArGoNa | Angle formed by the articulare, gonion and nasion | The inclination of mandibular ramus | 58.1° |
| NaGoMe | Angle formed by the nasion, gonion and menton | The inclination of mandibular body | 69.7° |
| NSAr | Angle formed by the nasion, sella and articulare | Extent of inclination of mandibular body | 124° |
| SArGo | Angle formed by the sella, articulare and gonion | Extent of anterior inclination of the mandible | 144.9° |
| Airway measurements | |||
| PNS-UPW | Distance between PNS and UPW | Nasopharyngeal airway space |
21.58 mm(M) 23.06 mm(W) |
| U-MPW | Distance between U and MPW | Upper oropharyngeal airway space |
9.92 mm(M) 10.22 mm(W) |
| V-LPW | Distance between V and LPW | Laryngeal airway space |
13.97 mm(M) 15.01 mm(W) |
| PAS | Width of the airway space along the Go-B line | Pharyngeal Airway Space | 10 mm |
| Soft tissue measurements | |||
| Surface angle | Angle formed by the G, Sn and Pos | Extent of protrusion of soft tissue | 12° |
| Nasolabial angle | Angle formed by the Prn, Sn and Ls |
Relationship between the upper lip and columella Nasi |
102° |
| Upper lip length | Linear distance between perpendiculars drawn to SN-POS from Sn and Stms respectively | Length of upper lip | 21.5 mm |
| Lower lip length | Linear distance between perpendiculars drawn to SN-POS from Mes and Stmi respectively | Length of lower lip | 47 mm |
| Upper lip protrusion | Linear distance between UL and Sn-Pos | Extent of protrusion of upper lip | 3 mm |
| Lower lip protrusion | Linear distance between LL and Sn-Pos | Extent of protrusion of lower lip | 4.2 mm |
| H angle | Angle formed by the Pos-UL and NB | Relationship between the soft tissue chin and lip | 10° |
Statistical analysis
Independent samples t-test was conducted to compare the difference between the measured index and the standard value, using SPSS software (version 22.0) for statistical analysis of measurements consistent with normality and homogeneity of variance. The significance level was set at 0.05.
Results
According to the cervical vertebral maturation assessment, there were 45 CS1 cases, 33 CS2 cases, 21 CS3 cases, 9 CS4 cases, 12 CS5 cases, and 0 case of CS6.
Maxillofacial hard tissue measurements
As shown in Table 2, SNB, GoGn and ArGoNa from CS1 through CS5 were below the standard values and had statistical significance. The measured values of ArGo and SNA were below the standard values and were statistically significant, only in CS1 and CS2 stages. The measured values of NGoMe (interval CS1–CS5), SN-MP (interval CS1–CS4), SN-PP (interval CS1–CS4), PP-MP (interval CS1–CS3) and SN-GoGn (interval CS1–CS2) were above the standard values and statistically significant. There was no statistical significance in other hard tissue measurements indexes of the maxillofacial region.
Table 2.
Measurements of hard tissue indexes at different cervical vertebral maturation stage
| Item | CS1 | CS2 | CS3 | CS4 | CS5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ¯X ± S | P value | ¯X ± S | P value | ¯X ± S | P value | ¯X ± S | P value | ¯X ± S | P value | |
| SNA | 79.75 ± 3.42 | 0.000 | 80.73 ± 2.36 | 0.004 | 81.60 ± 2.76 | 0.512 | 80.13 ± 3.67 | 0.165 | 81..00 ± 2.64 | 0.215 |
| SNB | 74.69 ± 5.05 | 0.000 | 74.59 ± 4.70 | 0.000 | 75.82 ± 5.51 | 0000 | 76.40 ± 3.18 | 0.003 | 78.21 ± 3.78 | 0.031 |
| ANB | 4.53 ± 2.27 | 0.000 | 5.36 ± 1.97 | 0.000 | 5.72 ± 5.20 | 0.027 | 3.73 ± 2.91 | 0.473 | 2.81 ± 1.72 | 0.715 |
| Y | 71.83 ± 4.79 | 0.000 | 72.22 ± 2.91 | 0.000 | 70.28 ± 5.45 | 0.012 | 72.56 ± 3.14 | 0.001 | 70.29 ± 4.07 | 0.017 |
| SN-MP | 39.13 ± 4.79 | 0.000 | 39.16 ± 4.36 | 0.000 | 36.94 ± 3.46 | 0.000 | 37.63 ± 3.85 | 0.007 | 35.43 ± 7.49 | 0.286 |
| FH-MP | 29.69 ± 4.21 | 0.000 | 29.82 ± 3.85 | 0.000 | 29.21 ± 3.88 | 0.001 | 28.33 ± 3,78 | 0.090 | 27.64 ± 6.76 | 0.393 |
| SN-Ar | 123.80 ± 5.23 | 0.796 | 124.42 ± 5.16 | 0.647 | 120.93 ± 9.10 | 0.137 | 123.52 ± 6.26 | 0.825 | 118.47 ± 11.47 | 0.123 |
| SArGo | 151.01 ± 5.67 | 0.000 | 150.44 ± 6.12 | 0.000 | 152.65 ± 5.27 | 0.000 | 153.00 ± 4.78 | 0.001 | 152.86 ± 4.04 | 0.000 |
| ArGo | 34.57 ± 3.41 | 0.000 | 35.29 ± 3.53 | 0.000 | 37.72 ± 3.97 | 0.083 | 38.61 ± 4.62 | 0.665 | 39.75 ± 4.62 | 0.742 |
| PP-MP | 28.72 ± 4.83 | 0.000 | 29.84 ± 4.53 | 0.000 | 27.91 ± 4.16 | 0.004 | 27.20 ± 4.34 | 0.167 | 25.27 ± 6.48 | 0.887 |
| SN-PP | 10.48 ± 2.69 | 0.000 | 9.32 ± 3.08 | 0.019 | 8.95 ± 1.89 | 0.032 | 9.44 ± 0.68 | 0.000 | 10.13 ± 3.53 | 0.060 |
| SN-GoGn | 36.4215 ± 4.75 | 0.000 | 36.43 ± 4.13 | 0.000 | 34.23 ± 3.47 | 0.119 | 34.96 ± 3.85 | 0.165 | 32.85 ± 7.31 | 0.944 |
| ArGoNa | 47.68 ± 3.99 | 0.000 | 48.18 ± 3.95 | 0.000 | 46.37 ± 3.87 | 0.000 | 45.62 ± 3.77 | 0.000 | 45.85 ± 3.82 | 0.000 |
| NaGoMe | 76.64 ± 4.62 | 0.000 | 76.07 ± 4.79 | 0.000 | 75.12 ± 4.65 | 0.000 | 75.21 ± 4.84 | 0.009 | 75.43 ± 6.19 | 0.008 |
| GoGn | 65.41 ± 5.60 | 0.000 | 65.65 ± 7.22 | 0.000 | 69.49 ± 6.90 | 0.001 | 67.34 ± 9.91 | 0.041 | 71.33 ± 4.5 | 0.010 |
Maxillofacial soft tissue measurements
As shown in Table 3, among soft tissue measurement indexes, H angle, lower lip length and upper lip protrusion were above the standard values with statistically significance from CS1 through CS5. The upper lip length (interval CS1–CS4), the lower lip protrusion (interval CS1–CS3), and surface Angle (interval CS2–CS3) were above the standard values with statistical significance. The nasolabial angle was above the standard value with statistical significance in the CS2 stage.
Table 3.
Measurements of soft tissue indexes at different cervical vertebral maturation stage
| Item | CS1 | CS2 | CS3 | CS4 | CS5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ¯X ± S | P value | ¯X ± S | P value | ¯X ± S | P value | ¯X ± S | P value | ¯X ± S | P value | |
| Surface angle | 14.72 ± 9.79 | 0.069 | 16.5274 ± 6.92 | 0.001 | 15.88 ± 6.35 | 0.011 | 15.82 ± 7.38 | 0.159 | 11.07 ± 4.74 | 0.511 |
| Nasolabial angle | 103.2193 ± 8.25 | 0.327 | 97.70 ± 11.12 | 0.034 | 102.44 ± 10.65 | 0.852 | 96.73 ± 15.43 | 0.335 | 101.30 ± 8.17 | 0.773 |
| Upper lip protrusion | 7.02 ± 1.85 | 0.000 | 9.62 ± 8.39 | 0.000 | 8.76 ± 6.51 | 0.001 | 11.23 ± 10.27 | 0.043 | 6.31 ± 1.62 | 0.000 |
| Lower lip protrusion | 5.72 ± 2.50 | 0.000 | 6.31 ± 1.84 | 0.000 | 6.27 ± 2.25 | 0.000 | 6.29 ± 3.34 | 0.097 | 5.27 ± 2.62 | 0.185 |
| Upper lip length | 18.73 ± 1.75 | 0.000 | 19.31 ± 1.74 | 0.000 | 19.82 ± 1.92 | 0.001 | 20.49 ± 2.54 | 0.268 | 19.6.9 ± 2.14 | 0.014 |
| Lower lip length | 38.47 ± 3.90 | 0.000 | 39.23 ± 4.10 | 0.000 | 40.84 ± 4.93 | 0.000 | 40.89 ± 4.77 | 0.005 | 4208 ± 4.66 | 0.004 |
| H angle | 20.30 ± 4.24 | 0.000 | 21.48 ± 3.36 | 0.000 | 21.28 ± 2.90 | 0.000 | 20.61 ± 5.50 | 0.000 | 17.93 ± 3.71 | 0.000 |
| PAS | 11.95 ± 3.77 | 0.001 | 12.26 ± 3.21 | 0.000 | 11.27 ± 3.82 | 0.144 | 11.04 ± 3.18 | 0.354 | 11.96 ± 2.69 | 0.028 |
Airway measurements
As shown in Table 4, PAS was above the standard value with statistical significance in CS1, CS2 and CS5. Regarding gender, the male PNS-UPW was below the standard value with statistically significance from CS1 to CS3, and the male U-MPW was above the standard value with statistically significance only in CS2. The male V-LPW was not statistically significant in the whole stage. The female PNS-UPW was below the standard value with statistical significance in the whole stage of CS1-CS5, and the female U-MPW and V-LPW were below the standard values with statistically significant only in the CS1 stage.
Table 4.
Measurements of airway indexes at different cervical vertebral maturation stage
| Item | CS1 | CS2 | CS3 | CS4 | CS5 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| male | P | female | P | male | P | female | P | male | P | female | P | male | P | female | P | male | P | female | P | |
| PNS-UPW | 15.28 ± 4.53 | 0.000 | 14.77 ± 3.0 | 0.000 | 17.32 ± 5.76 | 0.006 | 16.19 ± 4.13 | 0.000 | 16.42 ± 4.61 | 0.010 | 16.34 ± 4.97 | 0.001 | 17.77 ± 1.70 | 0.061 | 12.92 ± 3.47 | 0.001 | 19.07 ± 5.70 | 0.330 | 15.57 ± 3.69 | 0.004 |
| U-MPW | 10.58 ± 2.99 | 0.339 | 9.87 ± 2.94 | 0.048 | 10.75 ± 1.71 | 0.001 | 9.88 ± 2.51 | 0.609 | 9.88 ± 2.77 | 0.965 | 9.13 ± 2.10 | 0.099 | 10.63 ± 2.47 | 0.667 | 9.92 ± 1.07 | 0.518 | 10.32 ± 2.82 | 0.745 | 10.23 ± 1.87 | 0.987 |
| V-LPW | 12.98 ± 3.79 | 0.258 | 13.10 ± 3.37 | 0.009 | 13.69 ± 3.58 | 0.743 | 13.51 ± 3.54 | 0.122 | 12.87 ± 3.65 | 0.392 | 12.72 ± 4.20 | 0.085 | 12.93 ± 5.41 | 0.772 | 12.93 ± 3.00 | 0.151 | 16.12 ± 3.37 | 0.180 | 15.07 ± 3.65 | 0.971 |
Discussion
According to the review, studies on the effects of mouth breathing on craniofacial morphology and airway development in children were mainly based on cross-sectional data. They took mouth breathing children in a certain age stage or multiple age stages as the research objects [29]. Previous review articles suggested the correlation between dental development and skeletal maturation was strong, and CVM was a reliable method in predicting the pubertal growth spurt [30, 31]. There is a lack of study about craniofacial morphology and airway development in children and adolescents with mouth breathing based on the cervical vertebral maturation method.
In this study, included mouth breathing children and adolescents were classified into six stages (CS1–CS6) according to the cervical vertebral maturation method. The cephalometric analysis found that the maxillary protraction of mouth breathing children and adolescents was during CS1 and CS2, and the mandibular deficiency was throughout the whole stage from CS1 to CS5, reflected in the underdevelopment of the mandible body and the inferior-posterior rotation of the mandible. The inferior-posterior rotation of the maxilla was also observed in the stage from CS1 through CS4. Our first hypothesis has been accepted. The maxillary growth peak began at the quantitative cervical maturity stage 1 (QCVM Stage I), but then slowed down, while the maximum growth of mandible began at quantitative cervical maturity stage 2(QCVM Stage II), followed by quantitative cervical maturity stage 1(QCVM Stage I), suggesting that the peak of maxilla was earlier than that of mandible, and the growth period of mandible was longer than that of maxilla [32, 33]. Bisham proposed that the mandibular angle of adults shrunk substantially during the growth period, with a superior-anterior rotation [34]. These conclusions were consistent with our study results, indicating that mouth breathing had an important and varying impact on the maxilla and mandible development.
Regarding facial soft tissue development, the increasing trend of surface Angle in mouth breathing children and adolescents existed in CS2 and CS3 stages. The decrease of nasolabial angle only occurred during CS2. The increase of upper lip protrusion and the decrease of upper and lower lip length basically run through the whole period of children and adolescents' growth (CS1–CS5). Our second hypothesis has been accepted. This can be explained by the fact that the equilibrium effect between the lips and the teeth is lost as mouth breathing brings out the proclination of the upper anterior incisors and the parting of lips [35]. It was different from the previous conclusion that in the process of growth and development, the skeletal profile protrusion gradually decreased, and the soft tissue profile basically remained constant, verifying that the development of soft tissue had little correlation with the underlying hard tissue [36].
Regarding airway development, the nasopharyngeal airway in female mouth breathing children and adolescents was significantly narrowed throughout the whole period of CS1–CS5. The nasopharyngeal and upper oropharyngeal airway narrowing in male mouth breathing children and adolescents, and the upper oropharyngeal airway narrowing in female mouth breathing children and adolescents only existed in the developmental stage before CS3. The third hypothesis has been accepted. There was no statistical significance in the change of laryngopharynx airway in male mouth breathing children and adolescents. Female mouth breathing children and adolescents laryngopharynx airway was below standard value, only in CS1 period.
In conclusion, the effect of mouth breathing on the maxilla and mandibular ramus in children and adolescents mainly exists in the early growth and development. The shortening of mandibular body length and posteroinferior mandibular rotation were observed during the whole development of mouth breathing children and adolescents. The effect of mouth breathing on the upper lip protrusion, the shortening of upper and lower lip length and the retrogenia existed in each growth and development stage. Female nasopharyngeal stenosis was more likely to be affected than male at all stages of growth and development.
It is significant to stress the caution that should be taken while interpreting the results presented in this study, for the lack of control group and its limitations as a cross-sectional study regarding growth analysis, which is lack of sensitivity to individual variability. And the results may be varied by region on account of genetics and nutrition. Thus, it is suggested that longitudinal studies are performed in different populations, investigating the changes in hard tissue, soft tissue, and airway measurements between cervical vertebral maturation stages of mouth breathing children and adolescents.
Conclusions
The effect of mouth breathing on maxilla and mandibular ramus mainly existed in the early growth and development stage, while the effect on lip could exist through the whole growth and development stage. With regard to nasopharyngeal airway narrowing, females were more susceptible to oral-respiratory effects than males.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- CVM
Cervical vertebrae maturation
- CS
Cervical stage
Author contributions
JL and ZZ are the co-first author, contributing equally to this work. LZ and ZZ obtained funding. JL, YH, and ZZ designed the study. LZ, JL and ZZ collected the data. JL and BD were involved in data cleaning, and analysis of data. JL drafted the manuscript. JL and ZZ contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by Chongqing Talent Program: Innovative leading talents (Medical field, CQYC20210303384); Chongqing Medical Scientific Research Project (2018ZDXM020); Qingdao Key Health Discipline Development Fund (2020–2022).
Availability of data and materials
All data generated or analyzed during this study are included in the article.
Declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the ethical committee of the Stomatological Hospital of Chongqing Medical University. Written informed consent was obtained from guardian participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiahua Li and Ziyi Zhao these authors contributed equally to the article
References
- 1.Valera F, Travitzki L, Mattar S, Matsumoto M, Elias A, Anselmo-Lima W. Muscular, functional and orthodontic changes in pre school children with enlarged adenoids and tonsils. Int J Pediatr Otorhinolaryngol. 2003;67(7):761–770. doi: 10.1016/S0165-5876(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Bansal A, Asopa K. Prevalence of oral habits among eleven to thirteen years old children in Jaipur. Int J Clin Pediatr Dent. 2015;8(3):208–210. doi: 10.5005/jp-journals-10005-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi H, Tada S, Nakanishi Y, Kawaminami S, Shin T, Tabata R, Yuasa S, Shimizu N, Kohno M, Tsuchiya A, et al. Association between mouth breathing and atopic dermatitis in japanese children 2–6 years old: a population-based cross-sectional study. PLoS ONE. 2015;10(4):e0125916. doi: 10.1371/journal.pone.0125916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trabalon M, Schaal B. It takes a mouth to eat and a nose to breathe: abnormal oral respiration affects neonates' oral competence and systemic adaptation. Int J Pediatr. 2012;2012:207605. doi: 10.1155/2012/207605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grippaudo C, Paolantonio E, Antonini G, Saulle R, La Torre G, Deli R. Association between oral habits, mouth breathing and malocclusion. Acta Otorhinolaryngol Italica Organo Ufficiale Della Societa Italiana di Otorinolaringologia e Chirurgia Cervico-Facciale. 2016;36(5):386–394. doi: 10.14639/0392-100X-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhopatkar A, Bhatia S, Rock P. An investigation into the relationship between the cranial base angle and malocclusion. Angle Orthod. 2002;72(5):456–463. doi: 10.1043/0003-3219(2002)072<0456:AIITRB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Stahl F, Baccetti T, Franchi L, McNamara J. Longitudinal growth changes in untreated subjects with class II division 1 malocclusion. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod. 2008;134(1):125–137. doi: 10.1016/j.ajodo.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 8.D'Ascanio L, Lancione C, Pompa G, Rebuffini E, Mansi N, Manzini M. Craniofacial growth in children with nasal septum deviation: a cephalometric comparative study. Int J Pediatr Otorhinolaryngol. 2010;74(10):1180–1183. doi: 10.1016/j.ijporl.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Agostinho H, Furtado I, Silva F, Ustrell Torrent J. Cephalometric evaluation of children with allergic rhinitis and mouth breathing. Acta Med Port. 2015;28(3):316–321. doi: 10.20344/amp.5556. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz ICL, Orta PB. Comparison of cephalometric patterns in mouth breathing and nose breathing children. Int J Pediatr Otorhinolaryngol. 2014;78(7):1167–1172. doi: 10.1016/j.ijporl.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Juliano M, Machado M, Carvalho L, Prado L. do Prado G: Mouth breathing children have cephalometric patterns similar to those of adult patients with obstructive sleep apnea syndrome. Arq Neuropsiquiatr. 2009;67:860–865. doi: 10.1590/S0004-282X2009000500015. [DOI] [PubMed] [Google Scholar]
- 12.Juliano M, Machado M, de Carvalho L, Zancanella E, Santos G, do Prado L, do Prado G. Polysomnographic findings are associated with cephalometric measurements in mouth-breathing children. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2009;5(6):554–561. [PMC free article] [PubMed] [Google Scholar]
- 13.Juliano M, Machado M, Carvalho L, Santos G, Zancanella E, Prado L, Prado G. Obstructive sleep apnea prevents the expected difference in craniofacial growth of boys and girls. Arq Neuropsiquiatr. 2013;71(1):18–24. doi: 10.1590/S0004-282X2013000100005. [DOI] [PubMed] [Google Scholar]
- 14.Mattar S, Valera F, Faria G, Matsumoto M, Anselmo-Lima W. Changes in facial morphology after adenotonsillectomy in mouth-breathing children. Int J Pediatr Dent. 2011;21(5):389–396. doi: 10.1111/j.1365-263X.2011.01117.x. [DOI] [PubMed] [Google Scholar]
- 15.Lessa F, Enoki C, Feres M, Valera F, Lima W, Matsumoto M. Breathing mode influence in craniofacial development. Braz J Otorhinolaryngol. 2005;71(2):156–160. doi: 10.1016/S1808-8694(15)31304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi R, Rossi N, Rossi N, Yamashita H, Pignatari S. Dentofacial characteristics of oral breathers in different ages: a retrospective case-control study. Prog Orthod. 2015;16:23. doi: 10.1186/s40510-015-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambi-Rocha A, Cabrera-Domínguez M, Domínguez-Reyes A. Breathing mode influence on craniofacial development and head posture. Jornal de Pediatria. 2018;94(2):123–130. doi: 10.1016/j.jped.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Cheng M, Enlow D, Papsidero M, Broadbent B, Oyen O, Sabat M. Developmental effects of impaired breathing in the face of the growing child. Angle Orthod. 1988;58(4):309–320. doi: 10.1043/0003-3219(1988)058<0309:DEOIBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Souki B, Lopes P, Pereira T, Franco L, Becker H, Oliveira D. Mouth breathing children and cephalometric pattern: does the stage of dental development matter? Int J Pediatr Otorhinolaryngol. 2012;76(6):837–841. doi: 10.1016/j.ijporl.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 20.Harari D, Redlich M, Miri S, Hamud T, Gross M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope. 2010;120(10):2089–2093. doi: 10.1002/lary.20991. [DOI] [PubMed] [Google Scholar]
- 21.Minars M, Burch J, Masella R, Meister M. Predicting skeletal maturation using cervical vertebrae. Today's FDA Off Mon J Fla Dent Assoc. 2003;15(10):17–19. [PubMed] [Google Scholar]
- 22.Ilich J, Hangartner T, Skugor M, Roche A, Goel P, Matkovic V. Skeletal age as a determinant of bone mass in preadolescent females. Skeletal Radiol. 1996;25(5):431–439. doi: 10.1007/s002560050111. [DOI] [PubMed] [Google Scholar]
- 23.Franchi L, Baccetti T, McNamara J. Mandibular growth as related to cervical vertebral maturation and body height. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod. 2000;118(3):335–340. doi: 10.1067/mod.2000.107009. [DOI] [PubMed] [Google Scholar]
- 24.Fishman L. Can cephalometric x-rays of the cervical column be used instead of hand-wrist x-rays to determine patient's maturational age? Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod. 2002;122(1):18A–19A. doi: 10.1067/mod.2002.126148. [DOI] [PubMed] [Google Scholar]
- 25.Hassel B, Farman A. Skeletal maturation evaluation using cervical vertebrae. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod. 1995;107(1):58–66. doi: 10.1016/S0889-5406(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 26.García-Fernandez P, Torre H, Flores L, Rea J. The cervical vertebrae as maturational indicators. J Clin Orthod JCO. 1998;32(4):221–225. [PubMed] [Google Scholar]
- 27.Arat M, Köklü A, Ozdiler E, Rübendüz M, Erdoğan B. Craniofacial growth and skeletal maturation: a mixed longitudinal study. Eur J Orthod. 2001;23(4):355–361. doi: 10.1093/ejo/23.4.355. [DOI] [PubMed] [Google Scholar]
- 28.O'Reilly M, Yanniello G. Mandibular growth changes and maturation of cervical vertebrae–a longitudinal cephalometric study. Angle Orthod. 1988;58(2):179–184. doi: 10.1043/0003-3219(1988)058<0179:MGCAMO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z, Zheng L, Huang X, Li C, Liu J, Hu Y. Effects of mouth breathing on facial skeletal development in children: a systematic review and meta-analysis. BMC Oral Health. 2021;21(1):108. doi: 10.1186/s12903-021-01458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bittencourt M, Cericato G, Franco A, Girão R, Lima A, Paranhos L. Accuracy of dental development for estimating the pubertal growth spurt in comparison to skeletal development: a systematic review and meta-analysis. Dentomaxillofac Radiol. 2018;47(4):20170362. doi: 10.1259/dmfr.20170362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cericato G, Bittencourt M, Paranhos L. Validity of the assessment method of skeletal maturation by cervical vertebrae: a systematic review and meta-analysis. Dentomaxillofac Radiol. 2015;44(4):20140270. doi: 10.1259/dmfr.20140270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong R, Alkhal H, Rabie A. Use of cervical vertebral maturation to determine skeletal age. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod. 2009;136(4):484.e481–486. doi: 10.1016/j.ajodo.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Liu J, Xu T, Lin J. Longitudinal study of relative growth rates of the maxilla and the mandible according to quantitative cervical vertebral maturation. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod. 2010;137(6):736731–736738. doi: 10.1016/j.ajodo.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Bishara S, Peterson L, Bishara E. Changes in facial dimensions and relationships between the ages of 5 and 25 years. Am J Orthod. 1984;85(3):238–252. doi: 10.1016/0002-9416(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 35.Linder-Aronson S: Effects of adenoidectomy on dentition and nasopharynx. Trans Eur Orthod Soc 1972:177–186. [PubMed]
- 36.Genecov J, Sinclair P, Dechow P. Development of the nose and soft tissue profile. Angle Orthod. 1990;60(3):191–198. doi: 10.1043/0003-3219(1990)060<0191:DOTNAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the article.