Figure 2.
Nsp10 induced refolding of nsp14 ExoN lid subdomain
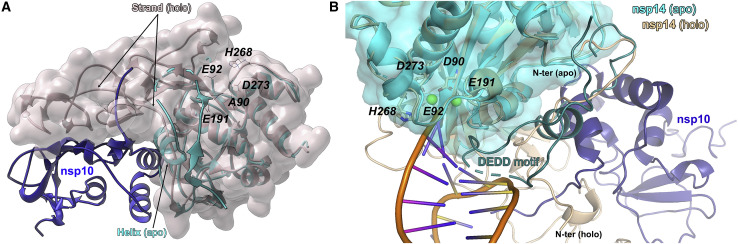
(A) Nsp10 (navy blue, ribbon)/Nsp14 (gray, surface) complex overlaid on apo-nsp14 (cyan). The N-terminal lid of nsp14 refolds upon binding of nsp10 as exemplified by Thr50-Met58 region assuming a helical structure in apo-nsp14 and forming a strand of a β sheet indicated in nsp10/nsp14 complex. In the apo structure, the lid occludes nsp10-binding site at the surface of nsp14.
(B) The RNA interaction site of nsp10 (navy blue)/nsp14 (beige) complex (7N0B) is sterically occluded by lid subdomain in apo-nsp14 structure (cyan), providing a rationale for the negligible exonuclease activity of nsp14 in the absence of nsp10. RNA (orange). The apo-nsp14 loop, occluding the RNA-binding site, is denoted in dark cyan (dotted line).
See also Figures S2 and S3.