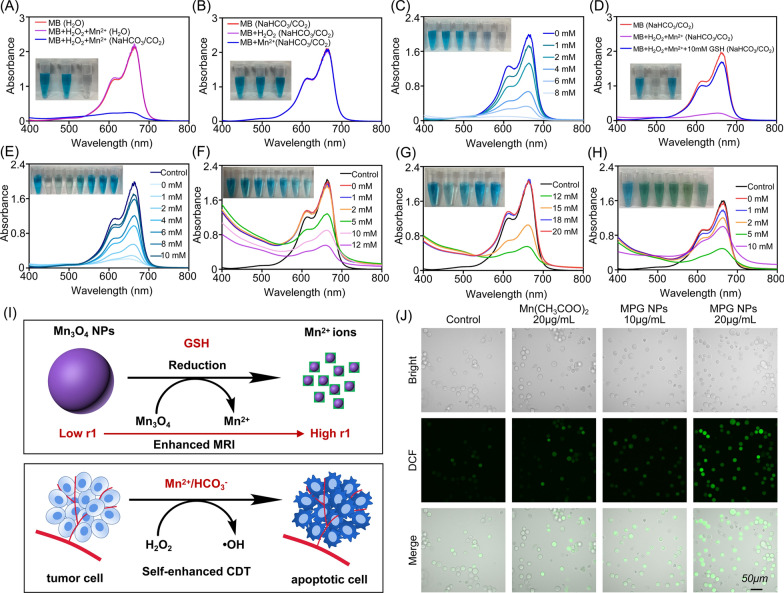
Fig. 4.
In vitro chemodynamic activity of MPG NPs. A UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ in different solutions. B UV-visible absorption spectra and photo (inset) of MB in the presence of Mn2+ and H2O2 alone. C UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ when the concentration of H2O2 gradually increases. D UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ with or without GSH. E UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ when the concentration of GSH gradually increases. F UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ (Mn3O4@PEG) when the concentration of GSH gradually increases. G UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ (Mn3O4@PEG) when the concentration of GSH gradually increases. H UV-visible absorption spectra and photo (inset) of MB degradation induced by Mn2+ (MPG NPs) when the concentration of GSH gradually increases. I Schematic diagram of MPG NPs reacting with GSH to produce Mn2+ and converting endogenous H2O2 into ·OH. J DCF fluorescence of SGC 7901 ADR with or without MPG NPs