FIGURE 2.
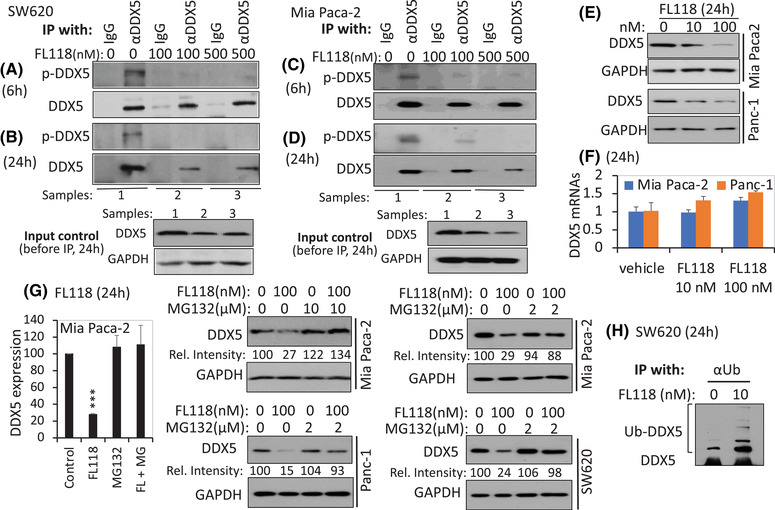
FL118 induces dephosphorylation and inhibition of the DDX5 protein. (A), (C) FL118 treatment for 6 h eliminates DDX5 phosphorylation on tyrosine (Y) residues in both SW620 cells (A) and Mia Paca‐2 cells (C). (B), (D) FL118 treatment for 24 h not only maintains the elimination of DDX5 Y phosphorylation but also inhibits DDX5 proteins in both SW620 cells (B) and Mia Paca‐2 cells (D). Colorectal cancer (CRC) SW620 cells and Pancreatic ductal adenocarcinoma (PDAC) Mia Paca‐2 cells with and without FL118 treatment for 6 h (A, C) or 24 h (B, D) as shown; cells were then analysed by immunoprecipitation (IP) with anti‐DDX5 antibody (αDDX5) or control IgG, followed by western blots with anti‐phospho‐tyrosine‐specific antibodies and αDDX5. The input controls shown in the bottom panel of (B) and (D) are 10% of cell lysates before IP. (E), (F) FL118 inhibits the DDX5 protein but not its mRNA. PDAC Panc‐1 and MiaPaca2 cells were treated with and without FL118 as shown. Cells were then analysed using western blots with DDX5 antibodies (E) or quantitative real‐time RT‐PCR (F). Data in (F) are the mean ± SD from three tests. (G) The proteasome inhibitor MG132 reverses FL118‐mediated degradation of the DDX5 protein. Mia Paca‐2, Panc‐1 and SW620 cells were treated with and without FL118 in the presence and absence of MG132 for 24 h as shown. Cells were then analysed using western blots with DDX5 antibody. The relative intensity (Rel. inten) was labelled in each western blot result. The histogram on the far‐left panel in (G) is the quantification of DDX5 protein bands (normalised to the GAPDH internal control) from two Mia Paca‐2 cell replicates. ***p value < .001. (H) FL118 treatment increases DDX5 ubiquitination. SW620 cells were treated with and without FL118 as shown. Cells were then analysed by IP with anti‐ubiquitin antibody, followed by western blots with αDDX5 antibodies. GAPDH in (B), (D), (E) and (G) is the internal protein loading control.
