Background:
Hemorrhagic and ischemic magnetic resonance imaging lesions as well as the more recently described decrease in vasomotor reactivity have been suggested as possible biomarkers for cerebral amyloid angiopathy (CAA). Analyses of these markers have been primarily cross-sectional during the symptomatic phase of the disease, with little data on their longitudinal progression, particularly in the presymptomatic phase of the disease when it may be most responsive to treatment. We used the unique opportunity provided by studying Dutch-type hereditary cerebral amyloid angiopathy (D-CAA) to determine longitudinal progression of CAA biomarkers during the presymptomatic as well as the symptomatic phase of the disease.
Methods:
In this longitudinal case-control study, magnetic resonance imaging markers and cognitive performance were assessed at baseline and after ≈4 years in 10 presymptomatic and 6 symptomatic D-CAA mutation carriers and 20 control subjects. These magnetic resonance imaging markers included hemorrhagic and ischemic manifestations, measurements of cerebral blood flow, and vasomotor reactivity to visual stimulation.
Results:
In presymptomatic D-CAA mutations carriers, vasomotor reactivity showed a decline over time for blood-oxygen-level–dependent amplitude (P=0.011) and prolongation of time to peak (P<0.001). In contrast, no significant changes in hemorrhagic markers, ischemic markers, cerebral blood flow, and cognition were found. In symptomatic D-CAA mutation carriers, the number of intracerebral hemorrhages increased over the 4-year period (P=0.007).
Conclusions:
Our findings indicate that in the presymptomatic phase of D-CAA, cerebrovascular reactivity measured by the blood-oxygen-level–dependent amplitude and time to peak to visual stimulation progressively worsens and can thus be regarded as a disease progression marker. In the symptomatic phase, the most salient marker of progression appears to be recurrent intracerebral hemorrhage.
Keywords: biomarker, cerebral amyloid angiopathy, hemorrhage, magnetic resonance imaging, mutation
Cerebral amyloid angiopathy (CAA) is a common type of small vessel disease in the brain that is caused by deposition of amyloid-β in small to medium-sized cerebral blood vessels and leptomeningeal arteries.1–3 CAA is an important cause of spontaneous lobar intracerebral hemorrhage (ICH) in the elderly and a contributor to age-related cognitive decline and dementia. The modified Boston criteria allow the diagnosis of possible or probable CAA in living persons based on the location and presence of (small) hemorrhagic lesions in the brain. Still, a definite diagnosis of CAA requires histological or postmortem evaluation.4 Population-based autopsy data indicate a CAA prevalence of 20% to 40% in nondemented elderly populations, which increases to 50% to 60% in demented elderly populations.5 In Alzheimer disease, CAA is identified in up to 98% of the cases when sought diligently.6 Unfortunately, to date, there is no disease-specific CAA treatment, and management of CAA patients is focused on the prevention of recurrent hemorrhagic events.7 In this respect, the development of disease-specific CAA treatment is severely hindered by the absence of early biomarkers reflecting disease progression.
Current magnetic resonance imaging (MRI) markers of CAA include both hemorrhagic and ischemic changes. Hemorrhagic manifestations include the presence of lobar cerebral microbleeds, ICHs, cortical superficial siderosis, and convexity subarachnoid hemorrhages.8,9 Ischemic manifestations include greater volumes of white matter hyperintensities (WMHs),10,11 cortical microinfarcts,11 or acute ischemic brain lesions.12,13 More recently, the presence of MRI-visible dilated perivascular spaces in the centrum semiovale (DPVS-CSO)14–18 and decreased vasomotor reactivity19–21 have also been put forward as CAA marker. Analyses of these markers have been primarily cross-sectional during the symptomatic phase of the disease, however, with little data on how their longitudinal progression, particularly in the presymptomatic phase of the disease when it may be most responsive to treatment. In symptomatic patients with CAA, it was suggested that progression of white matter lesions, hemorrhages, or worsening of cortical superficial siderosis could be a biomarker for assessing disease severity.10,22
Dutch-type hereditary CAA (D-CAA, also called hereditary cerebral hemorrhage with amyloidosis–Dutch type), an autosomal dominant form of CAA, can be used as disease model to study CAA with minimal confounding comorbidities associated with aging, other small vessel diseases, and potential other neurodegenerative disorders. In D-CAA, a Glu693Gln point mutation leads to extensive amyloid-β deposition in the meningocortical arterioles. This occurs without parenchymal deposition of amyloid-β plaques as in Alzheimer disease.23,24 In D-CAA the chemical composition and underlying pathology of the amyloid deposits, as well as the hemorrhagic and ischemic radiological manifestations, are similar to that in sporadic CAA.25,26 The development of symptoms (recurrent ICH and cognitive impairment) in D-CAA patients occurs relatively early in life, usually between the age of 50 and 60 years. That is in contrast to sporadic CAA, which is mainly seen in elderly over 65 years old. By using D-CAA as a disease model, the progression of MRI markers can be established, even in the presymptomatic phase of the disease without a large influence of age-related effects. This allows the search for an early biomarker that progresses over time, even before the disease becomes symptomatic.
Previously, we reported classic markers and potential new markers in D-CAA mutation carriers analyzed at a single cross-sectional timepoint.21,27 The aim of the present study is to establish the longitudinal progression of these CAA markers in presymptomatic and symptomatic D-CAA mutation carriers and to search for early biomarkers for disease progression in the presymptomatic group. We hypothesize that vasomotor reactivity, previously shown to be decreased in presymptomatic D-CAA,21 worsens over time and therefore could be a candidate biomarker for demonstrating effects on disease progression during the presymptomatic phase of CAA.
Methods
Our study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement. For a completed Strengthening the Reporting of Observational Studies in Epidemiology checklist please see Supplemental Material.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request (S.van_Rooden@lumc.nl).
Participants
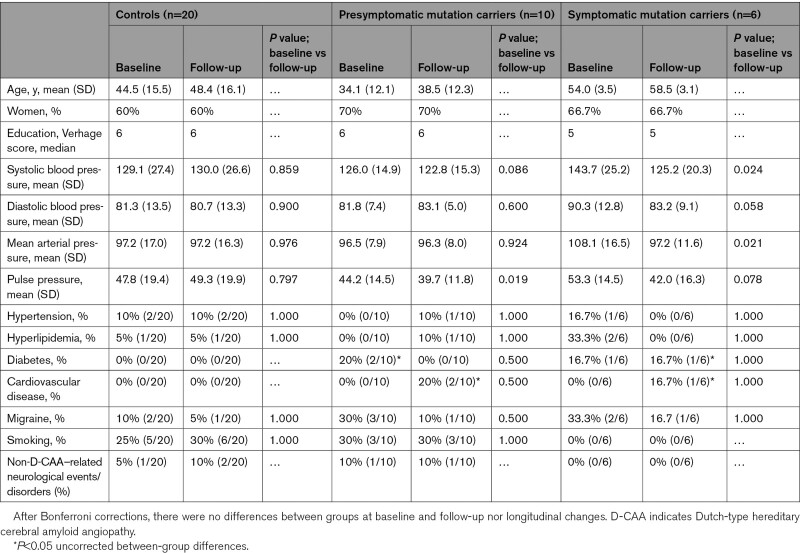
We performed this longitudinal case-control study between April 2017 and July 2018, at the Department of Radiology of the Leiden University Medical Center (Leiden, the Netherlands). For a patient flow diagram, please see Figure S1) Subjects enrolled in the baseline study21,27 were selected via the hereditary cerebral hemorrhage with amyloidosis–Dutch type patient association in Katwijk (the Netherlands) and the outpatient clinic of the Department of Neurology of the Leiden University Medical Center. Control subjects were selected from individuals at risk for D-CAA but who tested genetically negative and from subject spouses, family or friends. The general practitioners of all participants who were enrolled in the baseline study,21,27 performed between May 2012 and October 2013, were approached to check whether participants were still alive. All living participants were invited for a follow-up visit. At baseline, 60 participants were included: 27 D-CAA mutations carriers (12 presymptomatic and 15 symptomatic) and 33 control subjects. Presymptomatic was defined as having no symptomatic ICH in medical history. All participants enrolled in the study underwent genetic testing. Detailed inclusion criteria have been described previously.21,27 At follow-up 36 participants were included: 10 presymptomatic D-CAA mutation carriers (83% from baseline), 6 symptomatic D-CAA mutation carriers (40% from baseline), and 20 control subjects (61% from baseline). The presymptomatic mutation carriers had a mean age of 38.5 years (SD, 12.3) at follow-up, 3 were male, and 7 were female. The symptomatic mutation carriers had a mean age of 58.5 years (SD, 3.1), 2 male and 4 female. Controls had a mean age of 48.4 years (SD, 16.1), 8 male, and 12 female. The medical ethics committee of the Leiden University Medical Center approved the study and written informed consent was obtained from all subjects. The procedures followed were in accordance with institutional guidelines.
Magnetic Resonance Imaging
Image Acquisition
Imaging was performed on the same MRI scanner (3T Philips Medical Systems, Best, Netherlands) with exactly the same protocol as used at baseline.21 No relevant hardware or software upgrades have been made to the scanner in the 4-year follow-up interval. Three-dimensional T1-weighted images, T2-weighted images, fluid-attenuated inversion recovery scan, T2*-weighted images, pseudo continues arterial spin labeling scans, and visually stimulated blood-oxygen-level–dependent (BOLD) functional MRI scans were obtained with similar parameters as used at baseline.21,27 The visual stimulus consisted of 16 blocks of an 8 Hz flashing radial black and white checkerboard pattern for 20 seconds alternated with 28 seconds of gray screen, as described previously.19
Image Analysis/Processing
Hemorrhagic markers were assessed on T2*-weighted images. Lobar microbleeds (location as described by the Boston criteria28) were defined and scored, as described previously.29 ICHs were defined as parenchymal defects with evidence of hemosiderin in their wall. The number and presence of convexity subarachnoid hemorrhages were assessed described as a subarachnoid bleeding localized to the convexities of the brain.30 The presence of cortical superficial siderosis was described as linear residues of blood in the superficial layers of the cortex and classified as focal (≤3 sulci) or disseminated (≥4 sulci).8 DPVS were evaluated and assessed in the basal ganglia and CSO on axial T2-weighted images in line with STRIVE (standards for research into small vessel disease) definitions.31 Dilated perivascular spaces in the basal ganglia were rated according to a 1 to 4 point semiquantitative score, as previously described.32 DPVS-CSO were rated according to a 0 to 4 point semiquantitative score.33 Presence of lacunar infarcts was assessed using T2-weighted and fluid-attenuated inversion recovery images, as described earlier.34 The Fazekas scale (0–3) was calculated as the mean of periventricular white matter and deep white matter, both assessed using fluid-attenuated inversion recovery images according to a 4-point rating scale.35 The total brain MRI burden of small vessel disease in CAA (0–6), a compound score of lobar microbleeds, cortical superficial siderosis, DPVS-CSO, and WMHs, was scored.36 WMHs were defined and analyzed using a semiautomated and validated method using fluid-attenuated inversion recovery images, as described previously.37 Pseudo continuous ASL was used to determine the mean cerebral blood flow for the brain as whole and for 4 cortical areas separately (frontal, occipital, parietal, and temporal).21 BOLD functional MRI scans using a checkerboard stimulus were used to determine the BOLD amplitude, time to peak (TTP), and time to baseline (TTB), as described previously.19
Cognitive and Neuropsychiatric Function
All participants performed standardized neuropsychological testing at baseline and at follow-up. Global cognitive functioning was assessed using the Mini-Mental State Examination.38 Memory was evaluated using the Wechsler Memory Scale39 and Hopkins Verbal Learning Test.40 Psychomotor speed was assessed using Trail Making Test part A.41 For testing of executive function Trail Making Test part B,41 Digit Symbol Substitution test of the WAIS III (Wechsler Adult Intelligence Scale III)42 and Clock drawing were used. Language was evaluated using letter and category fluency43 and the Boston Naming Test.44 Also, neuropsychiatric tests included the assessment of apathy, using the Apathy Scale of Starkstein,45 and anxiety and depression, using the Hospital Anxiety and Depression Scale.46 Raw scores of the tests were used, except for the Wechsler Memory Scale memory quotient which was conventionally transformed into a scaled score.
Statistical Analysis
All statistical analyses were performed with the Statistical Package of Social Sciences (SPSS, version 25.0). Differences between controls and mutation carriers (presymptomatic and symptomatic) in demographic variables at baseline and follow-up were analyzed using univariate general linear modeling analysis for the normally distributed continuous variables, Mann-Whitney U tests for non-normally distributed continuous variables, and χ2 tests for categorical variables. To assess within-subject longitudinal change in demographic variables, paired-sample t test were used for normally distributed continuous variables and McNemar test for dichotomous variables. Bonferroni corrections for multiple comparison were applied.
Our primary objective is to assess within-subject longitudinal change in MRI parameters and cognitive and neuropsychiatric tests between baseline and follow-up. Therefore, paired-sample t tests were used for normally distributed continuous variables and Wilcoxon signed-rank tests for non-normally distributed continuous variables and categorical variables. In the symptomatic D-CAA group, only 3 subjects were included at follow-up with regard to vasomotor reactivity parameters; therefore, within-subject longitudinal change was not interpreted for these parameters.
For our secondary analysis, restricted to the presymptomatic mutation carrier group, we compared within-subject longitudinal change between presymptomatic mutation carriers and controls in candidate markers from our primary analysis. Within-subject change (delta scores) were calculated for each subject by subtracting the value at baseline from the value at follow-up. To compare the within-subject longitudinal change between groups, univariate general linear modeling analysis was used, with group and sex as fixed factor and age at baseline and interscan time interval as covariates.
Additionally, we performed multivariate linear regression model analyses to test for associations between candidate markers from our primary analysis and possible predictors at baseline. First, all possible predictors were entered into the model as independent variables. Second, a stepwise backward analysis was conducted to remove predictors with a low discriminant value.
All P values are expressed uncorrected. We did not use a correction for multiple comparison for our primary and secondary objectives due to our small and exploratory dataset where many of the outcomes covary with each other. In this respect, borderline significance were treated with care.
Results
Inclusion
Of the 60 participants at baseline, 4 symptomatic patients had died and 2 symptomatic patients were admitted to a nursing home during the follow-up period. Of the 54 remaining participants, 10 presymptomatic D-CAA mutation carriers, 6 symptomatic D-CAA mutation carriers, and 20 controls consented to participate in the follow-up study. Mutation carriers and control subjects who were included in the follow-up study did not differ in demographics from those who did not participate in the follow-up study (data not shown). Table 1 shows all demographic parameters of the participants that were included in the follow-up study. Mean follow-up time was 4.2±0.9 years. At baseline, 1 control subject reported a transient ischemic attack in medical history, and 1 presymptomatic mutation carrier reported traumatic brain injury. One control subject reported meningitis between baseline and follow-up. None of the presymptomatic mutation carriers reported neurological symptoms between baseline and follow-up. There were no differences in demographic data among presymptomatic D-CAA, symptomatic D-CAA, and controls, but age.
Table 1.
Demographics at Baseline and Follow-Up
MRI Measurements
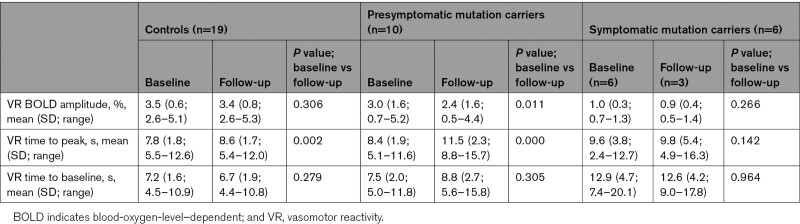
Vasomotor reactivity parameters at baseline and follow-up of the control, presymptomatic D-CAA, and symptomatic D-CAA group are shown in Table 2. In the symptomatic D-CAA group, 3 out of 6 subjects had missing vasomotor reactivity data. The other MRI characteristics at baseline and follow-up of the 3 groups are shown in Table S1–S3.
Table 2.
Vasomotor Reactivity Parameters at Baseline and Follow-Up
Longitudinal Changes
Control Subjects
In controls, WMHs increased over time: from 2.5 to 3.8 cm3 (P=0.021). DPVS in the CSO increased slightly over the 4-year period (P=0.046). Perfusion decreased over time in the occipital lobe from 42.1 to 39.1 mL/(100 g·min) (P=0.001), but not in the frontal, parietal, and temporal lobe or whole brain (Table S1). Concerning the vasomotor reactivity measurements, TTP was slightly prolonged after 4 years: from 7.8 to 8.6 s (P=0.002). BOLD amplitude and TTB were not altered (Table 2). Other MRI markers showed no significant differences over time.
Presymptomatic D-CAA Mutation Carriers
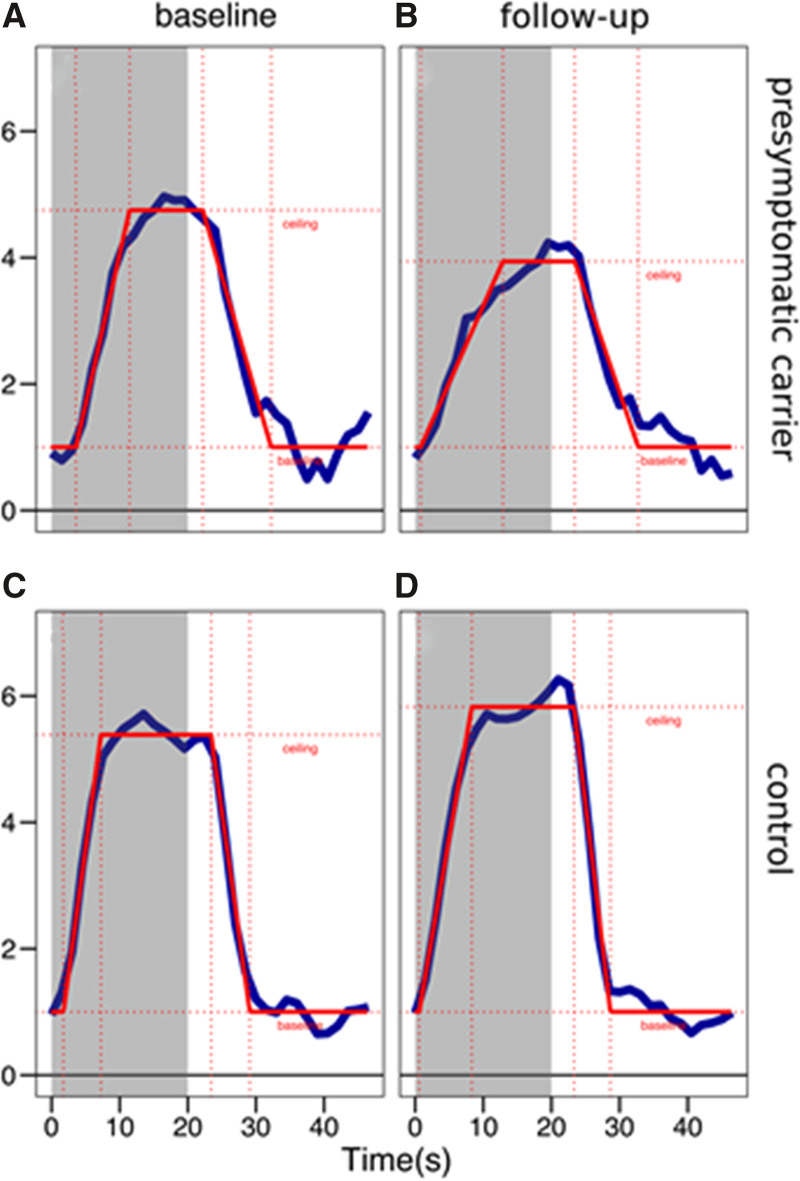
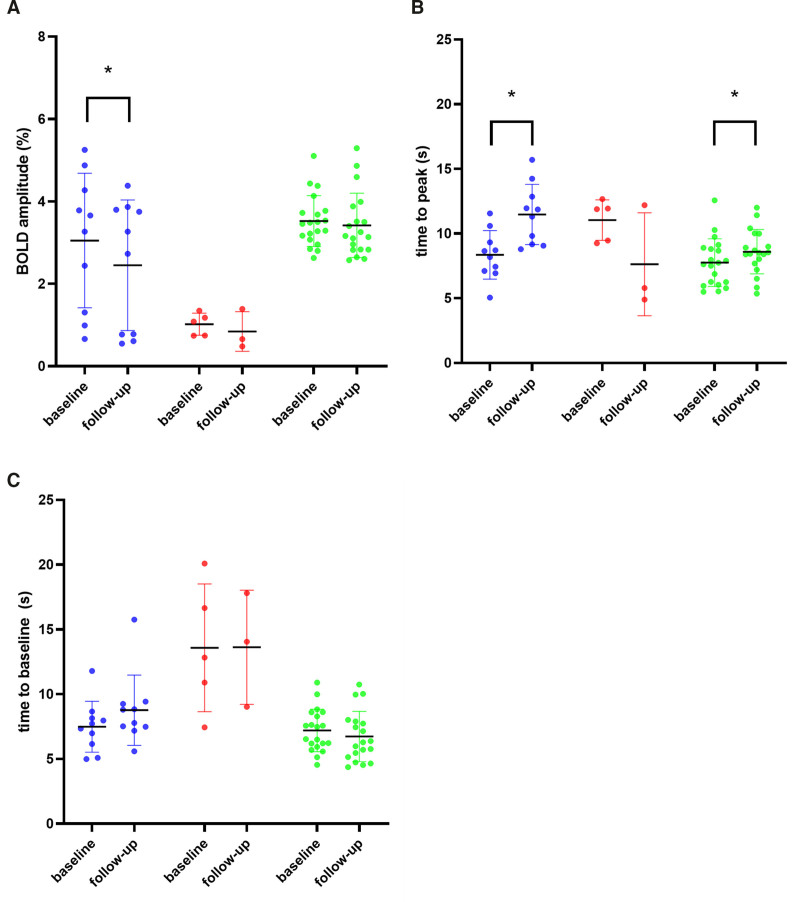
In presymptomatic D-CAA mutation carriers, vasomotor TTP was considerably prolonged over time: from 8.4 to 11.5 s (P<0.001) and BOLD amplitude demonstrated a small longitudinal decline (P=0.011). No longitudinal changes in TTB were observed (Table 2). Other MRI markers showed no significant differences over time (Table S2). Figure 1 shows a typical example of the shape of the hemodynamic response curves in the occipital lobe after checkerboard visual stimulation for a presymptomatic D-CAA mutation carrier and a control subject determined at baseline and follow-up. Figure 2 shows the individual data points for vasomotor reactivity measurements at baseline and follow-up.
Figure 1.
Shows a typical example of the shape of the hemodynamic response curves in the occipital lobe after checkerboard visual stimulation at baseline and after 4 y follow-up. The top figures represent the hemodynamic response curve of a presymptomatic mutation carrier at baseline (A) and follow-up (B). This particular person demonstrated a 16% decrease of blood-oxygen-level-dependent (BOLD) amplitude, a prolonged time to peak (+2.9 s), and a prolonged time to baseline (+1.4 s). The figures at the bottom represent the hemodynamic response curve of a controls subject at baseline (C) and follow-up (D).
Figure 2.
Shows the individual data points for vasomotor reactivity parameters at baseline and follow-up. Blood-oxygen-level-dependent (BOLD) amplitude (A), time to peak (B) and time to baseline (C) are shown. Presymptomatic Dutch-type hereditary cerebral amyloid angiopathy (D-CAA) mutation carriers are represented in blue, symptomatic mutation carriers in red, and controls in green. Significant longitudinal differences are shown with an asterisk.
Symptomatic D-CAA Mutation Carriers
In symptomatic D-CAA mutation carriers, the number of ICHs (P=0.007) and convexity subarachnoid hemorrhage (P=0.042) showed an increase over time. Cerebral blood flow was slightly decreased over time in the frontal lobe (P=0.025), parietal lobe (P=0.012), and whole brain (P=0.011). No cerebral blood flow changes were observed in the occipital or temporal lobe. Other MRI markers showed no significant differences over time (Table S3).
Secondary Analysis in Presymptomatic Mutation Carriers
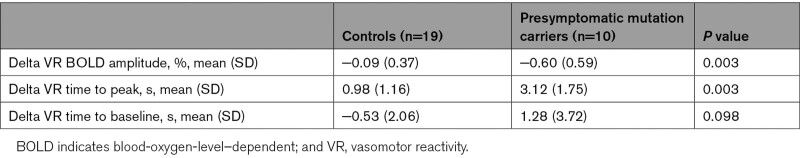
To further assess the changes over time in vasomotor reactivity parameters in presymptomatic mutation carriers, within-subject longitudinal changes (delta scores) were determined for the 3 vasomotor parameters: BOLD amplitude, TTP, and TTB. The within-subject longitudinal change of presymptomatic mutation carriers was compared with the change over time in controls. Table 3 shows the mean longitudinal change for presymptomatic mutation carriers and controls. Both groups showed a decline of BOLD amplitude over time; however, the decline in presymptomatic mutation carriers is larger than in controls (P=0.003). A similar finding was seen for TTP. Both groups showed a prolongation of TTP, but the change over time is larger for presymptomatic mutation carriers (P=0.003). No difference between groups was found for TTB.
Table 3.
Longitudinal Change in Vasomotor Reactivity Parameters
Moreover, a multivariate linear regression model with backward elimination was built to test the association between vasomotor reactivity progression and possible predictors (sex, interscan interval, and baseline parameters; age, WMHs, microbleeds, and DPVS-CSO). We found no associations between possible predictors and vasomotor reactivity progression (BOLD amplitude, TTP, and TTB).
Cognitive and Neuropsychiatric Function
Table S4 shows the corresponding neurocognitive data for the 3 groups at baseline and follow-up. Please see the Supplemental Material.
Longitudinal Changes
We found no significant change over time for cognitive or neuropsychiatric scores in any of the groups (controls, presymptomatic, and symptomatic D-CAA mutation carriers).
Discussion
The primary objective of this study was to assess within-subject longitudinal change in MRI parameters. This study shows that vasomotor reactivity, measured by changes in BOLD response after a visual checkerboard, is a sensitive marker for disease progression in the presymptomatic phase of D-CAA. In this phase of D-CAA, none of the other markers showed changes over time. In the symptomatic phase of the disease, only ICH severity showed worsening over time.
The most important finding of our study is that in the presymptomatic phase of D-CAA vasomotor reactivity parameters change over time. Our data show a decrease in BOLD amplitude and a prolongation of TTP over a 4-year period. Control subjects also show a prolongation of TTP over time; however, secondary analysis shows that the prolongation of TTP is substantially larger in presymptomatic mutation carriers than in controls. A previous study showed a decrease in BOLD amplitude in symptomatic patients with probable CAA over a 1-year period.47 Our study, however, is the first to describe such findings of progressive worsening of vasomotor reactivity in individuals with CAA without symptoms and largely without hemorrhagic markers both at baseline and follow-up. This suggests that the changes seen in BOLD response over time are primarily caused by changes in the vessel wall and possible accumulation of amyloid β, rather than structural damage due to hemorrhagic events. This is further supported by the lack of association between vasomotor reactivity progression and MRI markers at baseline. Cerebrovascular reactivity measured by the BOLD amplitude and TTP can thus be regarded as a disease progression marker in the presymptomatic phase of CAA.
In the symptomatic D-CAA group, only 3 subjects were included at follow-up with regard to vasomotor reactivity parameters; therefore, study power was too low to interpret findings in this group.
The second important finding of this study is that in the symptomatic phase of D-CAA, of all markers investigated, only ICH seemed to be a valuable progression maker. Our data showed a significant increase in ICHs over time in symptomatic D-CAA mutation carriers. This adds to the finding of a follow-up study in which 36% of sporadic CAA patients developed recurrent ICH in a mean follow-up time of 5 years after initial ICH.48 In presymptomatic D-CAA mutation carriers, no longitudinal differences in hemorrhagic markers were found. Our findings indicate that hemorrhagic markers, although valuable as disease markers for CAA in the symptomatic phase of the disease, appear to be less sensitive for disease progression in the presymptomatic phase. In the symptomatic phase, aggravation of ICHs appears to be the most sensitive hemorrhagic marker for disease progression.
We found no increase of WMHs over time in presymptomatic mutation carriers but did in controls subjects. Although, the absolute increase of WMHs was slightly larger in presymptomatic mutation carriers (5.3–7.3 mL) than in control subjects (2.5–3.8 mL). In symptomatic mutation carriers, we also found no significant increase of WMHs, but a large absolute increase was seen (48.7–67.8 mL). Our findings probably suffer from the relatively small sample size. Our previous study showed that WMHs were already more prevalent in the presymptomatic phase of D-CAA when compared with controls,27 and another study found a substantial increase of WMHs in patients with probable CAA over a 1-year period.10 Therefore, longitudinal research with larger groups is necessary to further assess the progression of WMHs, especially in the presymptomatic phase of CAA.
Our data show no longitudinal changes of the DPVS-CSO, Fazekas, or total small vessel disease score in CAA in presymptomatic and symptomatic mutation carriers. However, interpretation of the change over time of these MRI markers is severely hindered by a ceiling effect of the categorical scale used to assess them. In symptomatic mutation carriers, an increase over time could not be determined since almost all symptomatic mutation carriers were already in the highest categories at baseline. This reflects an important limitation of the use of categorical scales to assess disease progression. To draw conclusions about the value of DPVS-CSO as CAA biomarker, volumetric measurements as performed in an earlier study of Martinez Ramirez et al18 would be necessary. To summarize, our findings indicate that the semiquantitative measurements of DPVS-CSO, Fazekas, and total small vessel disease score in CAA are not sensitive for disease progression of CAA.
Perfusion markers showed no longitudinal change in presymptomatic mutation carriers. In symptomatic mutation carriers, we found a decrease in the frontal and parietal lobe and brain as whole. Perfusion in the occipital lobe decreased both in symptomatic D-CAA mutation carriers (33.0 to 28.9 mL/[100 g·min]) and controls (42.1–39.1 mL/[100 g·min]). However, only for the control group, this change was statistically significant, which probably is the result of the small sample size of the symptomatic group. Our findings of decreased perfusion at follow-up in symptomatic mutation carriers are in line with a previous study showing a decreased cerebral perfusion in patients with probable CAA.49 Reduced perfusion could be the result of progression of ICHs and reduced blood flow to damaged tissue. Taken together, our findings suggest that determination of perfusion is likely less suited for being a progression marker in de presymptomatic phase.
Our data do not show significant cognitive decline in the presymptomatic or symptomatic D-CAA mutation carriers, indicating that cognitive measurements cannot be regarded as sensitive markers for disease progression in CAA. The present study, although being a pilot study, suffers from especially the relatively small sample size. Our follow-up time of 4 years can be interpreted in 2 ways. However, for future drug trials, the follow-up time is too long to determine applicable progression markers. However, the follow-up time is too short to investigate converters into the symptomatic phase of the disease. Another limitation is that we investigated D-CAA, and the generalizability of our findings to sporadic CAA remains to be established as it occurs in older individuals in which there are comorbid changes caused by aging, other small vessel diseases, and neurodegenerative disorders.
In conclusion, our findings indicate that the most sensitive marker for disease progression in the presymptomatic phase of CAA is vasomotor reactivity measured by the BOLD amplitude and TTP after a visual stimulus. Hemorrhagic markers, although valuable as disease markers for symptomatic CAA, appear to be less sensitive for disease detection and progression in the presymptomatic phase of the disease. As the disease becomes symptomatic monitoring of the progression of ICHs is warranted.
Article Information
Sources of Funding
This work was supported by National Institutes of Health (NIH; R01 NS070834) and the Dutch cerebral amyloid angiopathy (CAA) foundation.
Disclosures
Dr Terwindt receives compensation from Eli Lilly and Company for consultant services; compensation from H. Lundbeck A S for consultant services; compensation from Allergan for consultant services; compensation from Teva Pharmaceutical Industries for consultant services; and compensation from Novartis Pharma for consultant services. Dr Gurol receives grants from Avid Radiopharmaceuticals, including grants from Boston Scientific Corporation and grants from Pfizer Canada Inc. The other authors report no conflicts.
Supplemental Material
STROBE Statement
Tables S1–S4
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BOLD
- blood-oxygen-level–dependent
- CAA
- cerebral amyloid angiopathy
- D-CAA
- Dutch-type hereditary cerebral amyloid angiopathy
- DPVS-CSO
- dilated perivascular spaces in the centrum semiovale
- ICH
- intracerebral hemorrhage
- TTB
- time to baseline
- TTP
- time to peak
- WMH
- white matter hyperintensity
This manuscript was sent to Liping Liu, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.035826
For Sources of Funding and Disclosures, see page 2013.
Contributor Information
Jeroen van der Grond, Email: j.van_der_grond@lumc.nl.
Jessie Lak, Email: jessielak@hotmail.com.
Annette van den Berg-Huysmans, Email: A.A.van_den_Berg-Huysmans@lumc.nl.
Gerda Labadie, Email: g.labadie@lumc.nl.
Gisela M. Terwindt, Email: g.m.terwindt@lumc.nl.
Marieke J.H. Wermer, Email: m.j.h.wermer@lumc.nl.
M. Edip Gurol, Email: megurol@partners.org.
Mark A. van Buchem, Email: m.a.van_buchem@lumc.nl.
Steven M. Greenberg, Email: sgreenberg@partners.org.
Sanneke van Rooden, Email: s.van_rooden@lumc.nl.
References
- 1.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277–290. doi: 10.1007/s00401-010-0652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghiso J, Frangione B. Cerebral amyloidosis, amyloid angiopathy, and their relationship to stroke and dementia. J Alzheimers Dis. 2001;3:65–73. doi: 10.3233/jad-2001-3110 [DOI] [PubMed] [Google Scholar]
- 3.Esiri MM, Wilcock GK. Cerebral amyloid angiopathy in dementia and old age. J Neurol Neurosurg Psychiatry. 1986;49:1221–1226. doi: 10.1136/jnnp.49.11.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston Criteria. Stroke. 2018;49:491–497. doi: 10.1161/STROKEAHA.117.016990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keage HA, Carare RO, Friedland RP, Ince PG, Love S, Nicoll JA, Wharton SB, Weller RO, Brayne C. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 2009;9:3. doi: 10.1186/1471-2377-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm (Vienna). 2002;109:813–836. doi: 10.1007/s007020200068 [DOI] [PubMed] [Google Scholar]
- 7.Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140:1829–1850. doi: 10.1093/brain/awx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linn J, Herms J, Dichgans M, Brückmann H, Fesl G, Freilinger T, Wiesmann M. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol. 2008;29:184–186. doi: 10.3174/ajnr.A0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman HH, Maia LF, Mackenzie IR, Forster BB, Martzke J, Woolfenden A. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke. 2008;39:2894–2897. doi: 10.1161/STROKEAHA.107.510826 [DOI] [PubMed] [Google Scholar]
- 10.Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, Greenberg SM, Smith EE. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haan J, Algra PR, Roos RA. Hereditary cerebral hemorrhage with amyloidosis-Dutch type. Clinical and computed tomographic analysis of 24 cases. Arch Neurol. 1990;47:649–653. doi: 10.1001/archneur.1990.00530060059018 [DOI] [PubMed] [Google Scholar]
- 12.Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Jäger HR, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134(pt 8):2376–2386. doi: 10.1093/brain/awr172 [DOI] [PubMed] [Google Scholar]
- 13.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83:124–137. doi: 10.1136/jnnp-2011-301308 [DOI] [PubMed] [Google Scholar]
- 14.Chen SJ, Tsai HH, Tsai LK, Tang SC, Lee BC, Liu HM, Yen RF, Jeng JS. Advances in cerebral amyloid angiopathy imaging. Ther Adv Neurol Disord. 2019;12:1756286419844113. doi: 10.1177/1756286419844113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, Gurol ME, Greenberg SM, Viswanathan A. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi: 10.1212/WNL.0b013e31828f1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, Xuereb J, Jäger R, Brandner S, Werring DJ. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. doi: 10.1212/01.wnl.0000438225.02729.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Jäger HR, Werring DJ. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013;84:624–629. doi: 10.1136/jnnp-2012-304434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Ramirez S, van Rooden S, Charidimou A, van Opstal AM, Wermer M, Gurol ME, Terwindt G, van der Grond J, Greenberg SM, van Buchem M, et al. Perivascular spaces volume in sporadic and hereditary (Dutch-Type) cerebral amyloid angiopathy. Stroke. 2018;49:1913–1919. doi: 10.1161/STROKEAHA.118.021137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez-Ramirez S, Schwab K, Rosand J, Viswanathan A, Salat DH, Polimeni JR, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peca S, McCreary CR, Donaldson E, Kumarpillai G, Shobha N, Sanchez K, Charlton A, Steinback CD, Beaudin AE, Flück D, et al. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013;81:1659–1665. doi: 10.1212/01.wnl.0000435291.49598.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Opstal AM, van Rooden S, van Harten T, Ghariq E, Labadie G, Fotiadis P, Gurol ME, Terwindt GM, Wermer MJH, van Buchem MA, et al. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: a case-control study. Lancet Neurol. 2017;16:115–122. doi: 10.1016/S1474-4422(16)30346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pongpitakmetha T, Fotiadis P, Pasi M, Boulouis G, Xiong L, Warren AD, Schwab KM, Rosand J, Gurol ME, Greenberg SM, et al. Cortical superficial siderosis progression in cerebral amyloid angiopathy: Prospective MRI study. Neurology. 2020;94:e1853–e1865. doi: 10.1212/WNL.0000000000009321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maat-Schieman M, Roos R, van Duinen S. Hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neuropathology. 2005;25:288–297. doi: 10.1111/j.1440-1789.2005.00631.x [DOI] [PubMed] [Google Scholar]
- 24.Maat-Schieman ML, Radder CM, van Duinen SG, Haan J, Roos RA. Hereditary cerebral hemorrhage with amyloidosis (Dutch): a model for congophilic plaque formation without neurofibrillary pathology. Acta Neuropathol. 1994;88:371–378. doi: 10.1007/BF00310382 [DOI] [PubMed] [Google Scholar]
- 25.Bornebroek M, Haan J, Maat-Schieman ML, Van Duinen SG, Roos RA. Hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D): I–A review of clinical, radiologic and genetic aspects. Brain Pathol. 1996;6:111–114. doi: 10.1111/j.1750-3639.1996.tb00793.x [DOI] [PubMed] [Google Scholar]
- 26.Maat-Schieman ML, van Duinen SG, Bornebroek M, Haan J, Roos RA. Hereditary cerebral hemorrhage with amyloidosis-Dutch type (HCHWA-D): II–A review of histopathological aspects. Brain Pathol. 1996;6:115–120. doi: 10.1111/j.1750-3639.1996.tb00794.x [DOI] [PubMed] [Google Scholar]
- 27.van Rooden S, van Opstal AM, Labadie G, Terwindt GM, Wermer MJ, Webb AG, Middelkoop HA, Greenberg SM, van der Grond J, van Buchem MA. Early magnetic resonance imaging and cognitive markers of hereditary cerebral amyloid angiopathy. Stroke. 2016;47:3041–3044. doi: 10.1161/STROKEAHA.116.014418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg SM. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology. 1998;51:690–694. doi: 10.1212/wnl.51.3.690 [DOI] [PubMed] [Google Scholar]
- 29.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Goddeau RP, Jr, Selim MH, Thomas A, Schlaug G, Alhazzani A, Searls DE, Caplan LR. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology. 2010;74:893–899. doi: 10.1212/WNL.0b013e3181d55efa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–2490. doi: 10.1161/STROKEAHA.110.591586 [DOI] [PubMed] [Google Scholar]
- 33.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–454. doi: 10.1161/STROKEAHA.109.564914 [DOI] [PubMed] [Google Scholar]
- 34.Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke. 2013;44:2995–2999. doi: 10.1161/STROKEAHA.113.002545 [DOI] [PubMed] [Google Scholar]
- 35.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 36.Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, Frosch M, Vashkevich A, Ayres A, Rosand J, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: an imaging-pathologic study of concept validation. JAMA Neurol. 2016;73:994–1001. doi: 10.1001/jamaneurol.2016.0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hafkemeijer A, Altmann-Schneider I, de Craen AJ, Slagboom PE, van der Grond J, Rombouts SA. Associations between age and gray matter volume in anatomical brain networks in middle-aged to older adults. Aging Cell. 2014;13:1068–1074. doi: 10.1111/acel.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 39.Wechsler D. A standardized memory scale for clinical use. J Psychol. 1945;19:87–95. doi: 10.1080/00223980.1945.9917223 [Google Scholar]
- 40.Brandt J. The hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. doi: 10.1080/13854049108403297 [Google Scholar]
- 41.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. doi: 10.2466/pms.1958.8.3.271 [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Scale-third edition. The Psychological Corporation Limited; 1997. [Google Scholar]
- 43.Lezak MD. Neuropsychological Assessment. Oxford University Press.; 1995. [Google Scholar]
- 44.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; 1983. [Google Scholar]
- 45.Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry. 2001;158:872–877. doi: 10.1176/appi.ajp.158.6.872 [DOI] [PubMed] [Google Scholar]
- 46.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 47.Switzer AR, McCreary C, Batool S, Stafford RB, Frayne R, Goodyear BG, Smith EE. Longitudinal decrease in blood oxygenation level dependent response in cerebral amyloid angiopathy. Neuroimage Clin. 2016;11:461–467. doi: 10.1016/j.nicl.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Etten ES, Gurol ME, van der Grond J, Haan J, Viswanathan A, Schwab KM, Ayres AM, Algra A, Rosand J, van Buchem MA, et al. Recurrent hemorrhage risk and mortality in hereditary and sporadic cerebral amyloid angiopathy. Neurology. 2016;87:1482–1487. doi: 10.1212/WNL.0000000000003181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung YA, O JH, Kim JY, Kim KJ, Ahn KJ. Hypoperfusion and ischemia in cerebral amyloid angiopathy documented by 99mTc-ECD brain perfusion SPECT. J Nucl Med. 2009;50:1969–1974. doi: 10.2967/jnumed.109.062315 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request (S.van_Rooden@lumc.nl).