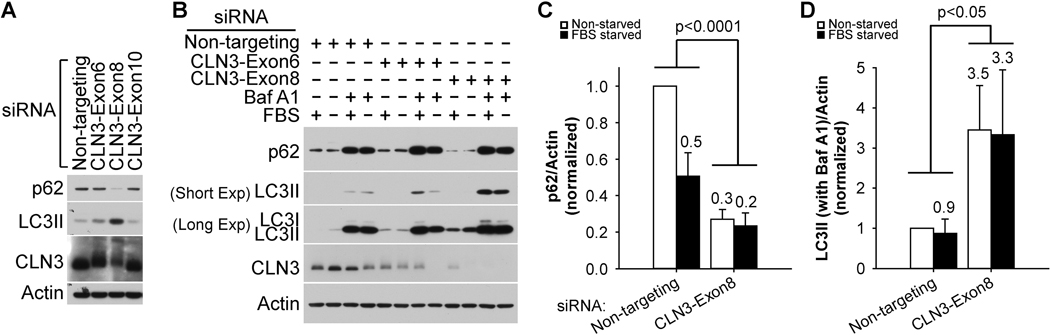
Figure 2. CLN3 deficiency led to increased autophagic flux in RPE-1 cells.
(A) The siRNA against CLN3 exon 8 (as compared to the siRNA against CLN3 exon 6 or exon 10, or non-targeting siRNA) was the most effective in reducing both CLN3 and p62 protein levels and in increasing LC3II levels. Of note, CLN3 protein ran as a 64–76 kDa band (Supplemental Figure 3). (B) Representative immunoblotting data showed that CLN3 siRNA treatment resulted in decreased p62 protein levels in the absence of bafilomycin A1 (Baf A1) and increased LC3II levels in the presence Baf A1 (200 nM, 18 h). (C) Quantification of immunoblotting results for p62 in the absence of Baf A1 showed decreased p62 protein levels upon CLN3 exon 8 siRNA (versus non-targeting siRNA) treatments. (D) Quantification of immunoblotting results for LC3II in the presence of Baf A1 showed increased LC3II protein levels upon CLN3 exon 8 siRNA (versus non-targeting siRNA) treatments. For both (C) and (D), cells were cultured under non-starved (open bars) or 18 h FBS-starved (closed bars) conditions. Statistics were carried out using two-way ANOVA and p values for comparing CLN3 exon 8 siRNA versus non-targeting siRNA were displayed. For p62, experiments were performed 5 times under non-starved conditions and 3 times under FBS-starved conditions, respectively. For LC3II, experiments were performed 4 times under non-starved conditions and 3 times under FBS-starved conditions, respectively.