OVERVIEW AND INTRODUCTION
With the implementation of Regulation (European Union [EU]) 2017/746 on in vitro diagnostic medical devices (IVDR), from May 26, 2022, onwards, the development and use of diagnostic tests will be governed by a vastly expanded and upgraded EU regulatory framework. We provide here an overview of the amended transition timelines, the role of notified bodies, EU reference laboratories, expert panels, and the Medical Device Coordination Group (MDCG). We also describe the implications of the IVDR for innovative laboratory medicine by explaining the exemption for in-house devices (IH-IVDs). Two key challenges faced by the academic diagnostic sector are: (1) the stipulation on equivalence of tests (article 5.5d), which poses a new condition for the use of IH-IVDs and (2) the gray area between CE marked in vitro diagnostics (CE-IVDs), modified CE-IVDs, Research Use Only (RUO) tests, and IH-IVDs. Furthermore, the results of a questionnaire on current diagnostic practice conducted by European medical societies collaborating in the BioMed Alliance indicate widespread use of IH-IVDs in diagnostic laboratories across Europe and emphasize the need for support and guidance to comply with the IVDR. Diagnostic equivalents of the European Reference Networks (ERNs) for rare diseases could help ensure affordable and equal access to specialized diagnostics across the EU. Concerted action by clinical and laboratory disciplines, regulators, industry, and patient organizations is needed to support the efficient and effective implementation of the IVDR in a way that preserves innovation and safeguards the quality, safety, and accessibility of innovative diagnostics.
Laboratory medicine plays an increasingly important part in medical decision-making at diagnosis and follow-up and evolution towards “Personalized” or “Precision” medicine in Hematology and other disciplines. It is, therefore, not surprising that this has long been an area with strict regulatory surveillance, particularly in specialties generating numerical results. These are more easily subject to evaluation of analytical performance parameters such as trueness (bias) and precision (repeatability and reproducibility), than descriptive, qualitative diagnostic specialties.
Within the EU, laboratory or in vitro diagnostic (IVD) tests were regulated by the 1998 Directive 98/79/EC on in vitro diagnostic medical devices (IVDD),1 which mainly governed pre-market production within the manufacturing sector of CE-IVD marked tests. The majority were self-declared by manufacturers, with EU-wide application, and only approximately 10% required certification by notified bodies, appointed by the national competent authorities in Member States. IVDD did not regulate use of laboratory-developed tests (LDT)/in-house devices (IH-IVD, referred to herein as IH-IVD as this is the term used in EU legislation), which are manufactured and used within the same health institution for medical purposes, in keeping with the EU principle of subsidiarity, and consequent national regulation.
In 2017, 2 new EU regulations were adopted: the Regulation (EU) 2017/746 on IVDR2 and the Regulation (EU) 2017/745 on medical devices (MDR). In contrast to Directives, Regulations are directly applicable in all Member States and do not need to be transposed into national legislation. This reduces the potential for divergent interpretation in different Member States. The IVDR requires more accurate description of intended use and clinical evidence and has strengthened post-market performance follow-up and post-market surveillance (in conjunction with notified bodies and competent authorities), all of which have been introduced for the benefit of the patient. As described in the guidance on general principles of clinical evidence for IVDs from the MDCG, the clinical evidence, including scientific validity, analytical performance and clinical performance data, needs to verify the safety and performance for all claims in the intended purpose.3,4 The IVDR, like the IVDD, still exempts IH-IVDs from most requirements applicable to CE-marked devices. However, for the first time, it provides a list of conditions for that exemption5 (see below), thereby opening an EU-wide regulatory dimension to development and use of innovative diagnostic tests. The IVDR will have major implications for the latter, some of which seem to have been poorly taken into consideration. This commentary will describe the IVDR regulatory environment and the implementation timelines based on a recent amendment. It will also present results of a questionnaire on current diagnostic practice, disseminated to European medical societies via the Biomed Alliance, with particular attention to the development and use of innovative tests. Appropriate concerted action should allow us to avoid the IVDR complicating innovation and instead support it from its early development steps onwards.
THE TRANSLATIONAL DIAGNOSTIC VALUE CHAIN
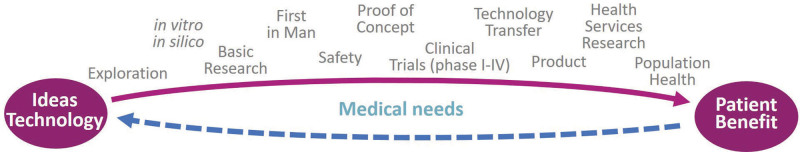
There will always be coexistence of CE-IVD tests provided by the manufacturing sector and IH-IVDs developed and used by the academic diagnostic sector,6 with complementary roles in the translational research value chain illustrated in Figure 1.
Figure 1.
The translational research value chain.
Ideally, development by academia will lead to transfer to the industrial sector of tests that meet criteria of economic viability for the latter, with appropriate recognition and compensation (eg, via royalties from licensed tests) to the former.3 Industrial transfer is unlikely to be the case for all diagnostic tests, particularly those that are rare and/or complex.3 Factors preventing transfer include, but are not limited to: analytical complexity and dependence on patient-derived material for controls; suitability for automation; algorithm dependence; lack of interest from manufacturers and a variety of national diagnostic and reimbursement practices that affect economic viability. There is also a tendency to maintain within the academic sector tests that lead, directly or indirectly, to establishment of the tissue and data banks that form the basis for future discovery and development. The real-world pertinence and relative clinical effectiveness of IVDR are evaluated by Health Technology Assessment (HTA) agencies. Depending on the country, these also perform the economic appraisal or provide the evidence used for cost-effectiveness and reimbursement decisions by payers. The new EU Regulation on HTA7 will provide a mechanism for harmonized, collaborative clinical assessments as well as joint scientific consultations and horizon scanning.
IVDR REGULATORY ENVIRONMENT
Succinctly, IVDR legislation2 classifies IVD tests into 4 classes, with increasing personal and public risk. Class A represents low individual and low public health risk, class B moderate individual and/or low public health risk, class C high individual and/or moderate public health risk, and class D high individual and high public health risk. Classes B, C, and D (and sterile class A devices) require notified body certification, thus representing a massive increase (from approximately 10% to 80% of tests) in the proportion of CE-IVD tests to be evaluated by a small number (6 in February 2022) of notified bodies.8,9 IVDD-compliant tests are not considered automatically compliant with the IVDR, that is, all such tests need reevaluation. Class C tests include many used in Hematology, such as screening, diagnosis, or staging of cancer, genetic testing, and companion diagnostics.
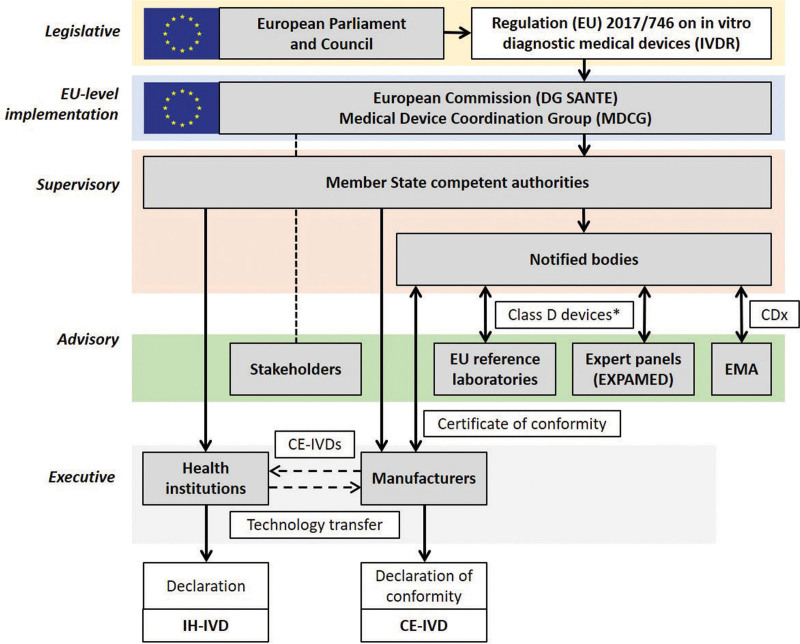
Class D devices cover essentially high-risk infectious agent testing (such as in blood transfusion or testing for life-threatening diseases) and blood/tissue compatibility testing. In addition to the overall assessment by a notified body, the performance of class D devices is to be verified by independent testing in EU reference laboratories, once those laboratories are designated by the European Commission. For novel class D devices, the performance evaluation has also to be reviewed by an IVD expert panel. Expert panels on MDs and IVDs (EXPAMED)10 have been developed by the Joint Research Centre, the European Commission’s internal scientific service, and will be managed by the European Medicines Agency (Figure 2). A European Database on Medical Devices (EUDAMED)12 containing information on devices on the market under MDR and IVDR is also under development, but few modules are available to date.
Figure 2.
The European IVDR regulatory environment with inclusion of the IH-IVD activities of health institutions. For details on CE-IVDs, see Cobbaert et al.11 EU level implementation of the IVDR, adopted by the European Parliament and the Council of the EU in 2017, is executed by the European Commission (DG SANTE) and the Medical Device Coordination Group (MDCG), which is chaired by the European Commission and consists of representatives of the Member State competent authorities. Stakeholders such as the BioMed Alliance and EFLM are invited to advise/comment. Supervision of notified bodies, health institutions, and economic operators such as IVD manufacturers is the primary task of the Member State competent authorities. For all IVDs except class A nonsterile devices, manufacturers need to submit their technical documentation to notified bodies for conformity assessment. For class D devices, consultation of EU reference laboratories and/or expert panels (coordinated by the EMA on behalf of the European Commission) can be part of this procedure (*expert panels are consulted when it is the first certification of that type of device and there are no common specifications; EU reference laboratories are involved when a relevant EU reference laboratory is designated; otherwise, it is not mandatory). For CDx, which are typically class C, consultation of the EMA (or a national medicines agency) is included in the conformity assessment procedure by the notified body. After approval, the notified body issues a certificate of conformity, allowing the manufacturer to CE mark the IVD and place it on the market for use in diagnostic patient care. In addition to such CE-IVD tests, health institution diagnostic laboratories can develop and use in-house devices (IH-IVDs), which need to meet a number of conditions and requirements specified in IVDR Article 5.5. Some IH-IVDs might become available for the broad diagnostic community as CE-IVDs after successful technology transfer. CDx = companion diagnostics; CE-IVDs = CE marked in vitro diagnostics; DG SANTE = Directorate-General for Health and Food Safety; EFLM = European Federation of Clinical Chemistry and Laboratory Medicine; EMA = European Medicines Agency; EU = European Union; EXPAMED = expert panel on medical devices and in vitro diagnostic devices; IH-IVDs = in-house in vitro diagnostics; IVD = in vitro diagnostic; IVDR = Regulation (EU) 2017/746 on in vitro diagnostic medical devices; MDCG = Medical Device Coordination Group.
Progress in implementation at the EU level is led by the IVD Working Group of the MDCG,13 which is composed of representatives of Member States and the European Commission. Relevant, representative stakeholders are invited to participate in regular meetings and to comment on consultative documents that are subsequently made public, in order to guide stakeholders on a range of topics.14
Readers interested in the details of IVDR implementation are invited to consult the BioMed Alliance statements15 and the references cited in this commentary.5,14,16,17 Given the very large number of diagnostic medical specialties, particularly within the academic sector, MDCG representation is assured by federative alliances; the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and the Biomedical Alliance in Europe (BioMed Alliance). EFLM represents many medical laboratory specialties and 40 national associations, particularly Clinical (Bio)Chemistry and General Laboratory Medicine. The BioMed Alliance represents a wide range of European medical specialty associations with an interest in medical research, including many with a diagnostic component, including Clinical/Biochemistry, Hematology, Pathology, Human Genetics (inherited and somatic), Immunology, Microbiology, and Reproductive Medicine. More information can be found on the webpage of the BioMed Alliance Task Force on IVDs.15
IVDR IMPLEMENTATION TIMELINES
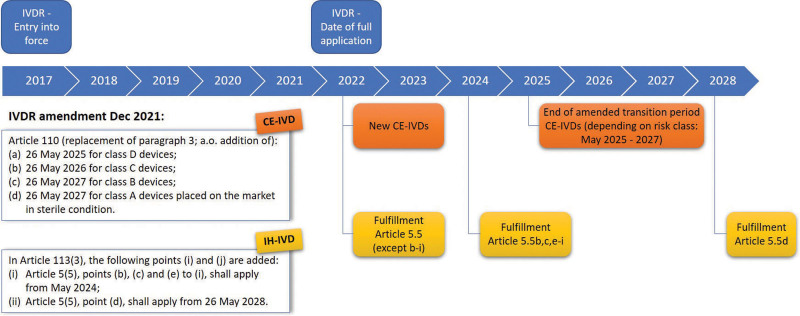
The implementation timelines initially envisaged for the MDR were May 26, 2020, and May 26, 2022, for the IVDR. The Covid pandemic led to the first change in timelines, pushing back the date of full application of the MDR to May 2021. This coincided with redeployment of regulatory/stakeholder IVDR efforts to development and appropriate regulation of CE-IVD kits for detection of SARS-CoV-2, following initial PCR test development by the academic diagnostic sector. The timelines for IVDR were challenging from the outset, but it increasingly became clear that operational concerted functioning of the EXPAMED panels, EU reference laboratories and the EUDAMED database by May 2022 would be difficult, even if provisions for functioning prior to EUDAMED availability for IVDR are specified in Article 113(f) of the IVDR. In addition, there was, and still is, a significant lack of notified bodies. This led to widespread fears that many, vital, CE-IVDs would (at least temporarily) disappear from the market throughout Europe in May 2022, with no possibility for the public sector to compensate with IH-IVDs,11 whose regulatory compliance requires increasing justification.5,16 Consequently, the decision18 by the Council and the European Parliament in December 2021 to adopt a European Commission proposal19 amending the IVDR implementation timelines, following widespread advocacy activity, was welcomed by both the manufacturing sector (represented principally by MedTech Europe) and by the BioMed Alliance and EFLM. The staggered, revised implementation timelines are shown in Figure 3.
Figure 3.
Timelines for revised phased IVDR implementation. The General Safety and Performance Requirements specified in Annex I as well as Article 5.5 (with the exception of 5.5b to i) are not mentioned in the amendment and are, as such, applicable from May 2022. CE-IVDs = CE marked in vitro diagnostics; IH-IVDs = in-house in vitro diagnostics; IVDR = Regulation (EU) 2017/746 on in vitro diagnostic medical devices.
It is to be hoped that this will allow the MDCG, national competent authorities, notified bodies, EXPAMED panels, EU reference laboratories, and EUDAMED database developers to prepare the regulatory infrastructures required for implementation of IVDR-mandated changes, with achievement of the hoped-for optimization of clinical benefit. It is also worth noting that the General Safety and Performance Requirements specified in Annex I of the IVDR are not mentioned in the amendment and are therefore still applicable from May 2022. Annex I consists of almost 200 single requirements governing risk management, performance evaluation/clinical evidence, information on the design and manufacture of the device, and instructions for use. As such, the revised timelines still require immediate preparation by the diagnostic and manufacturing sectors, as the EU infrastructures are being put in place. For the latter, for example, IVDD CE-IVDs will have to be certified, if belonging to one of the corresponding categories or, alternatively, have a declaration of conformity issued before May 26, 2022, in order to benefit from the extended transitional periods. New and significantly altered IVDs cannot make use of the extended transitional periods after that date.
IN-HOUSE DEVICES: AN EXEMPTION THAT COMES WITH OBLIGATIONS
IVDR Article 5.52,5 states that “with the exception of the relevant general safety and performance requirements set out in Annex I, the requirements of this Regulation shall not apply to devices manufactured and used only within health institutions established in the Union, provided that all of the following conditions are met.” The conditions for this IH-IVD exemption are listed in Article 5.5a–i.
Article 5.5a specifies that transferring (the physical parts of) IH-IVDs to other legal entities is not allowed, as did the IVDD. This does not prevent European collaborative groups from sharing laboratory protocols and information about technologies, tools and reagents.
Articles 5.5b,c require diagnostic labs to comply with the International Organization for Standardization standard EN ISO 15189, which specifies requirements for quality and competence in medical laboratories, or with applicable national provisions.
Articles 5.5d,e are extremely important as they refer to the justification of the use of IH-IVDs. In-house tests will only be allowed to be used, under the IVDR, if there is no equivalent CE-IVD kit on the market or when a target patient-group’s specific needs cannot be met at the appropriate level of performance by an equivalent CE-IVD. The deadline for demonstrating this has been postponed to 2028. Appropriate interpretation of clauses and the definition of terms such as “equivalent” and “patient-specific needs”20 is crucial, as they are the basis that should enable laboratories to compose an optimal portfolio of CE-IVDs and IH-IVDs. We argue that such interpretation is part of the responsibility of professional societies’ working groups, which have a clear understanding of the basis of evidence-based laboratory medicine (EBLM), including assessment of diagnostic research in a regulatory context. Such initiatives, in particular at local and national levels, are already ongoing. These should be aligned at an international level, wherever and whenever appropriate. The EUDAMED database, once fully functional, is expected to be the major source of information about equivalent devices available on the market. Since the deadline for manufacturers to CE mark class C devices under the IVDR is 2026, and Art. 5.5d will apply from 2028 onwards, Hematology laboratories should have at least 2 years to search for equivalent devices for most of their IH-IVDs, review them, and implement if applicable (a process that will have to be repeated periodically).
Article 5.5f requires diagnostic laboratories to generate a public declaration mentioning that IH-IVDs meet the General Safety and Performance Requirements of Annex I.2
Extra requirements for class D devices are stipulated in Article 5.5g,h. Member States may apply this provision also to class A, B, or C devices.
Article 5.5i concerns clinical review of experience and corrective actions by the health authority. Finally, the last sentence of Article 5 states that the IH-IVD exemption is only valid for devices that are not manufactured on an industrial scale. It is worth mentioning that laboratories should always check for national adaptations/additions. In Germany, for example, Article 5.5g,h will also be applicable for class A–C IH-IVDs.
Much of Article 5.5 does not differ significantly from currently required documentation for an ISO 15189 compliant laboratory,16 although the review of experience gained from clinical use will change from desirable practice to a regulatory requirement (Article 5.5i). It is of note that the IVDR is not an ISO standard and is consequently not evaluated by the national (ISO) accrediting bodies but by the (regulatory) national competent authorities (Figure 2). The ISO standard is much broader than the IVDR, but the latter adds to the ISO standard the extra IH-IVD-specific requirements mentioned above.
Most uncertainty and concerns within the public, and particularly the academic, diagnostic sector regarding Article 5.5 involve 2 issues: (1) Article 5.5d and viability of innovative diagnostics and (2) Definition of an IH-IVD and the gray area between CE-IVDs, use of modified CE-IVDs, use of RUO tests, and IH-IVDs.
In a more general context, it is widely recognized that the IVDR specifies what diagnostic laboratories must do, whereas it is up to diagnostic specialists to determine how this must be achieved. It is currently less clear who will provide the guidance and support on implementation of these changes and meet the inevitable costs of increased regulatory compliance. The manufacturing sector will fund the development and approval of CE-IVDs, but it will be up to the academic and/or public sector to do the same for IH-IVDs. There are some ongoing national and international efforts to help medical laboratories.16,17,21 One example is the AWMF (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften), a network of scientific medical societies in Germany that provides templates and further guidance on how to implement the IVDR in a laboratory.21,22 The European Scientific foundation for Laboratory Hemato-Oncology (ESLHO) has offered yearly IVDR workshops since 2020. Additionally, Q&As regarding the IVDR and resources such as video tutorials are freely available on the ESLHO website.23 The EFLM Working Group on Test Evaluation24 is preparing a practical understanding of study design principles and key data requirements needed to search or generate clinical evidence for each of the intended purposes (so beyond diagnostic accuracy studies only) to use and interpret medical test results.
ARTICLE 5.5D AND FUTURE VIABILITY OF INNOVATIVE DIAGNOSTICS
The reasoning behind the stipulation that the health institution must justify that the specific needs of a particular group of patients cannot be met or cannot be met at the appropriate level of performance, by an equivalent device available on the market, is open to speculation. Justifications include a higher benefit/harm ratio for the intended patient group based on, for example, improved clinical effectiveness and better inter-center reproducibility and reliability and increased possibilities for central compilation of results. Improved clinical effectiveness would include, as an example, rare variants of oncogenic fusion transcripts or mutations. While some guidance25 on equivalence for MDs exists, there is no guidance yet for how equivalence should be evaluated for IVD assays, nor when target patient-group’s specific needs are considered to be met at the appropriate level of performance. This must be clarified well in advance of the 2028 revised implementation date by diagnostic specialists with expertise in EBLM in an international and interdisciplinary setting and in alignment with national competent authorities. Properly designed studies aimed at identifying the optimal way to generate clinical evidence and evaluation of medical test inferiority, equivalence, and superiority are required.
It is also possible that at least part of the reasoning is to help stimulate the European biotechnology sector by encouraging academic specialists to intellectually protect and disseminate, via the manufacturing sector, their in-house developed tests. Data on the proportion of health sector patents that are eventually licensed suggest that this process could be improved. Whether the IVDR will help reduce the various limitations to dissemination of innovative diagnostics, over and above peer-reviewed publication, remains to be seen. Additional initiatives may be needed to empower test developers to commercialize their products or disseminate them in other ways.
Taken to its conclusion, if an IH-IVD is superior to comparable devices available on the market, there is, at least theoretically, public health responsibility to make such a test available to a maximum number of patients who are likely to benefit. These situations are pertinent to all diagnostic sectors but perhaps disproportionately concern niche tests, often performed by reference laboratories. Whether such tests are performed by the academic or the manufacturing sector has implications for tissue and sample banks for rare cancers, genetic disorders, subpopulations, and infectious pathogens. The common denominator here is their rarity. It is possible that the IVDR will provide impetus to, and justification for the creation of “European Diagnostic Reference Networks.” These would be complementary to, and linked with, the existing ERNs for rare diseases. In Hematology, IH-IVDs disproportionately involve genetic testing for malignancies and congenital disorders, often referred to as precision diagnostics. Appropriate Hematology diagnostic reference networks would logically complement current transverse investment within the EuroBloodNet ERN.26
The risk inherent to Article 5.5d is that of monopolies, whereby the existence on the market of a single IVD device will discourage optimization of, and competition by, alternative devices developed by the academic sector. This may well be associated to a risk of excessive costs, once monopolized, and to a failure to detect very rare abnormalities. Academia is well placed to challenge existing IVDs, mainly through access to annotated patient samples, and to design new standards of diagnostic care through IH-IVD optimization, including in partnership with the manufacturing sector. It is worth mentioning that judgment of equivalence will not be done by industry or notified bodies. It is up to the health institutions to judge if equivalent devices are available and to have documented justification for their use of IH-IVDs. The competent authorities will oversee the compliance of health institutions with respect to observation of Article 5.5. It is also important to leave sufficient time for laboratories to shift, when appropriate, from IH-IVD to CE-IVD tests, since this will necessitate instrumentation, logistics, organizational, and quality assessment changes, with their attendant costs.
To conclude, interpretation of IVDR Article 5.5d, in particular the provision regarding equivalence of tests, should be addressed carefully so as to preserve efficiency, safety, and lean principles in academic settings, in line with the rationale of the IVDR.
QUESTIONNAIRE BENCHMARKING CURRENT DIAGNOSTIC PRACTICES WITHIN THE EU
In order to evaluate the spectrum of tests performed by diagnostic laboratories, the Biomed Alliance distributed a questionnaire to its members, as well as through various communication channels, in July 2021 to October 2021. It did not attempt to assure exhaustivity or balanced representativity of responses or diagnostic specialties, for whom labels, and clustering tends to vary between countries. The full report can be found on the BioMed Alliance website.27
Two-hundred three responses were collected from medical laboratories in the EU and Norway, including at least 1 response from 25 of the 27 EU Member States. Variable representation by Member States was interpreted to reflect, in part, the existence and/or participation of a national umbrella pan-diagnostic medicine structure involving the academic sector. The majority of respondents were public or university hospital laboratories, but academic research laboratories and private and/or nonhospital laboratories were also represented. Respondents reported activities in a wide range of diagnostic specialties (41 in total), reflecting significant heterogeneity in identification or labeling of specialist activities. While these demonstrated considerable overlap, with possibilities for regrouping, this was not considered coherent with the nature of the questionnaire, other than for 23 respondents with 19 distinct labels.
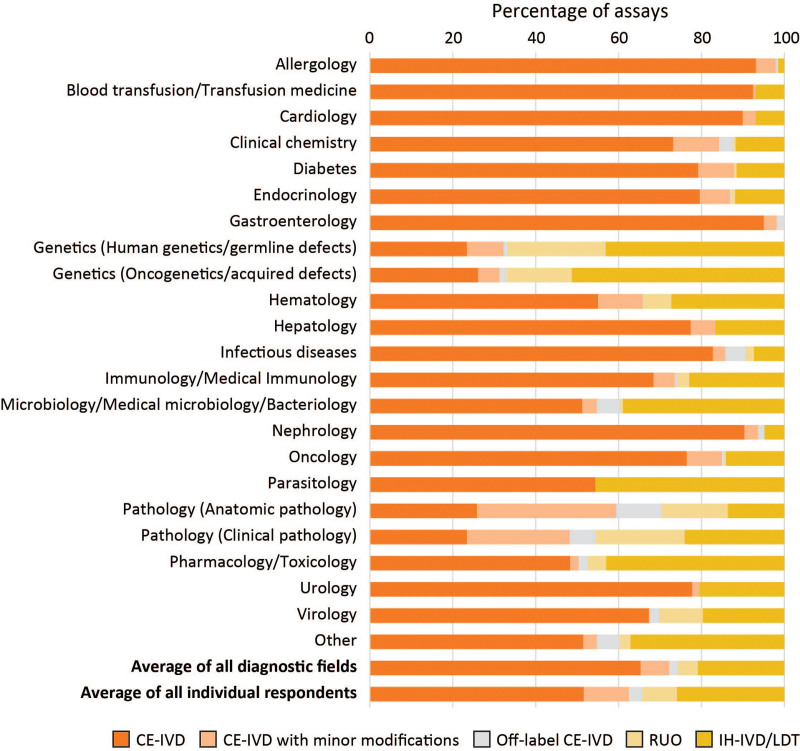
Overall, 150 laboratories replied for a total of approximately 30,000 (overlapping) IVDs. Test use at the interface between CE-IVDs and IH-IVDs was classified as “modified CE-IVD” when there were minor modifications not affecting safety or effectiveness, “off-label CE-IVD” when there were significant deviations from the intended purpose/instructions for use affecting clinical performance, such as use of different sample categories, or “RUO” when such kits were used for diagnostics under the responsibility of the diagnostic laboratory. The relative ratio of the different categories of IVDs used by laboratories from the indicated diagnostic fields was highly diverse (Figure 4). On average, the respondents had implemented 52% CE-IVDs, 14% modified/off-label CE-IVDs, 8% RUOs, and 26% IH-IVDs. The diagnostic fields with the highest percentage of IH-IVDs were Genetics, Microbiology/Parasitology, and Pharmacology/Toxicology. Hematology laboratories scored close to the average with 55% CE-IVDs, 11% modified/off-label CE-IVDs, 7% RUOs, and 27% IH-IVDs, although it is likely that some laboratories classified their hematological molecular tests within the genetics (germline or acquired) categories. Succinctly, IH-IVDs are likely to represent 25%–50% of Hematology tests, primarily determined by the proportion of molecular diagnostics performed.
Figure 4.
Average percentage of assays from 5 IVD categories used by respondents of the questionnaire (n = 150), per diagnostic field. (1) CE-IVDs used strictly according to the manufacturer’s IFU; (2) CE-IVDs with minor modifications; (3) Off-label CE-IVDs; (4) RUO kits; and (5) In-house devices (IH-IVDs)/LDTs. A detailed explanation of the 5 IVD categories can be can be found in the main text and the report on the BioMed Alliance website.27 CE-IVDs = CE marked in vitro diagnostics; IFU = instructions for use; IH-IVDs = in-house in vitro diagnostics; IVD = in vitro diagnostic; LDT = laboratory-developed test; RUO = Research Use Only.
These responses, albeit on a voluntary basis, serve to illustrate that CE-IVD tests used exclusively as indicated by manufacturers only correspond to one half of the type of tests used by the, predominantly academic, hospital-based diagnostic sector. It is, of course, worth noting that this corresponds to a much smaller proportion of total diagnostic test results, since the vast majority of high-throughput tests are CE-IVD and will remain so. This questionnaire also confirmed the need for revised implementation timelines (subsequently met by the publication of the IVDR amendment in December 202118), the widespread anticipation of increasing costs of diagnostics and a need for guidelines and support (standard documents, templates, examples, workshops) to facilitate adherence to the IVDR. Despite the amended timelines (Figure 3), there is a lot of work to be done, both before May 2022 (mainly fulfillment of Annex I) and in the following 2 years, in order to respect the transitional regulatory requirements demanded in the amended IVDR.
WILL THE COST OF IVDR BE OFFSET BY ITS BENEFITS?
While this is currently speculative, it seems clear that IVDR compliance will increase the cost of diagnostics. The IVDR obliges the manufacturer to perform more thorough evaluation and more extensive third-party independent evaluation, which will (1) improve the standards and performance of CE-IVD tests and (2) force the manufacturers to withdraw devices that have poor evidence of safety and performance. As such, the IVDR will improve the level of safety and performance of devices on the market in the EU and beyond. But the additional costs of respecting these requirements for both the manufacturing and diagnostic sectors will be significant. If, however, this is accompanied by optimized prescribing and appropriate concentration of expertise for highly specialized IH-IVD tests, within connected reference networks, increased unit costs could be associated with decreased overall consolidated costs of patient care, with a shift from therapeutic to diagnostic costs.
The number of IH-IVDs performed by a given laboratory is likely to reduce, given the additional workload inherent in proving superiority to a manufactured alternative. In the case of CE-IVDs, IVD manufacturers have a legal responsibility to document the clinical evidence and other IVDR requirements. Those from the innovative diagnostic sector who plan to continue to use and develop IH-IVDs should use the amended transition times wisely in order to document the benefit and clinical evidence in an appropriate way. If academic protection of innovative diagnostics is considered desirable, it will be necessary to fund and support alternative actions for IH-IVDs, in order to make sure that, for example, rare and/or specialized diagnostic tests across the spectrum of diagnostic disciplines continue to be offered to patients who need them. Ideally, the structures that provide such support would be European, given the rarity of this category of patients and the enormous potential of concerted diagnostic practice.
ERNs26,28 for rare diseases have achieved much in the holistic, public health structuring of prevention, care and education, as have the increasing number of European Comprehensive Cancer Institutes and infectious agent reference structures. European diagnostic equivalents could work in synergy with these structures and in cooperation with regulators, MedTech Europe and the IVD industry, clinical and laboratory medicine societies, patient organizations, etc., to ensure optimal, fair access to specialized diagnostics across the EU. Such structures must be created in collaboration with national umbrella diagnostic medical reference organizations. Countries such as the Netherlands, Belgium, and Germany have already shown the advantages of concerted, transpecialty action in preparing for the IVDR, by fostering collaboration between national competent authorities, accreditation bodies, and HTA structures. It is possible that the IVDR may set off concerted actions whose benefits go far beyond the regulatory injunctions and the, understandable, fear of “yet more bureaucracy.”
THE WAY FORWARD
Hopefully, the IVDR will improve patient management through better and safer commercial and in-house developed tests at acceptable costs. There is a clinical need for both CE-IVD and IH-IVD tests, as there is for modified CE-IVDs, RUO tests, and off-label use, as long as their benefit for patients is obvious or can be proven. Ideally, the IVD industry and research/academic labs would create a joint biomarker-to-test pipeline so that innovative diagnostics are first developed and applied locally, in healthcare institutions or diagnostic reference networks with specific expertise, and then taken over by IVD manufacturers above a certain production volume that allows cost-effective production and commercialization. In that case, access to innovative diagnostics—so essential for continuous optimization of patient care—remains guaranteed.
AUTHOR CONTRIBUTIONS
ID and BRL performed the questionnaire. LS collated information. All authors contributed to writing the article.
BIOMED ALLIANCE TASK FORCE ON IN VITRO DIAGNOSTICS
Active members include European Academy for Allergy and Clinical Immunology represented by Domingo Barber, Mohamed Shamji; European Society of Pathology represented by Aurelio Ariza, Dr Raed Al Dieri, Gerald Hoefler; European Society of Clinical Microbiology and Infectious Diseases represented by Emmanuelle Cambau; European Federation of Immunological Societies represented by Raivo Uibo; European Renal Association represented by Danilo Fliser; European Alliance of Associations for Rheumatology represented by Loreto Carmona; European Association of Urology represented by Anders Bjartell; European Academy of Neurology represented by Peter Van den Bergh; United European Gastroenterology represented by Tamara Matysiak-Budnik; and European Association for the Study of the Liver represented by Maria Buti.
DISCLOSURES
JJMvD reports an Educational Services Agreement from BD Biosciences (San José, CA) and a Scientific Advisor Agreement with Cytognos; all related fees and honoraria are not received personally but go to Leiden University Medical Center. JJMvD reports to have given an Educational Lecture for Amgen; the related honorarium was not received personally but was paid to University of Salamanca. JJMvD reports to be chairman of the EuroFlow Consortium, which receives royalties from licensed patents, which are collectively owned by the participants of the EuroFlow Foundation. These royalties are exclusively used for continuation of the EuroFlow collaboration and sustainability of the EuroFlow consortium. MB received personal fees from Incyte (advisory board) and Roche Pharma AG; financial support for reference diagnostics from Affimed, Amgen, and Regeneron; grants and personal fees from Amgen (advisory board, speakers bureau, travel support); and personal fees from Janssen (speakers bureau), all outside the submitted work. EM reports that she is president of the European Hematology Association and a BioMed Alliance board member. All the other authors have no conflicts of interest to disclose.
Footnotes
ID and BRL have contributed equally/shared first authorship.
The information and views set out in this article are those of the author(s) and do not necessarily reflect the official opinion of the European Commission.
Active Members of the BioMed Alliance Task Force on In Vitro Diagnostics are listed at the end of this publication.
REFERENCES
- 1.Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Official J Eur Commun. 1998;331:1–37. [Google Scholar]
- 2.Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Official J Eur Union. 2017;117:176–332. [Google Scholar]
- 3.Lubbers BR, van Dongen JJM. Position of European Consortia in the IVDR Era: Support for In-house Devices (IH-IVDs) and CE Marked IVDs (CE-IVDs). 9th ESLHO Symposium Abstract Book. 2020:33–46. Available at: https://eslho-public.s3.nl-ams.scw.cloud/Lubbers2020_94364d9779.pdf. Accessed March 5, 2022.
- 4.Medical Device Coordination Group. MDCG 2022-2 Guidance on General Principles of Clinical Evidence for In Vitro Diagnostic Medical Devices (IVDs). 2022. Available at: https://ec.europa.eu/health/system/files/2022-01/mdcg_2022-2_en.pdf. Accessed March 5, 2022.
- 5.Lubbers BR, Schilhabel A, Cobbaert CM, et al. The new EU regulation on in vitro diagnostic medical devices: implications and preparatory actions for diagnostic laboratories. HemaSphere. 2021;5:e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeersch P, Van Aelst T, Dequeker EMC. The new IVD Regulation 2017/746: a case study at a large university hospital laboratory in Belgium demonstrates the need for clarification on the degrees of freedom laboratories have to use lab-developed tests to improve patient care. Clin Chem Lab Med. 2020;59:101–106. [DOI] [PubMed] [Google Scholar]
- 7.Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU. Official J Eur Union. 2021;458:1–32. [Google Scholar]
- 8.European Commission. Nando (New Approach Notified and Designated Organisations) Information System: Notified Bodies Designated for the IVDR. Available at: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=35. Accessed March 5, 2022.
- 9.van Drongelen A, de Bruijn A, Pennings J, van der Maaden T. The impact of the new European IVD classification rules on the notified body involvement; a study on the IVDs registered in the Netherlands. RIVM Lett Rep. 2018;2018-0082:1–32. [Google Scholar]
- 10.European Commission. Medical Devices - Expert Panels. Available at: https://ec.europa.eu/health/medical-devices-expert-panels/overview_en. Accessed March 5, 2022.
- 11.Cobbaert C, Capoluongo ED, Vanstapel FJLA, et al. Implementation of the new EU IVD regulation – urgent initiatives are needed to avert impending crisis. Clin Chem Lab Med. 2022;60:33–43. [DOI] [PubMed] [Google Scholar]
- 12.European Commission. EUDAMED - European Database on Medical Devices. Available at: https://ec.europa.eu/tools/eudamed/#/screen/home. Accessed March 5, 2022.
- 13.European Commission. Medical Device Coordination Group Working Groups. Available at: https://ec.europa.eu/health/medical-devices-dialogue-between-interested-parties/medical-device-coordination-group-working-groups_en. Accessed March 5, 2022.
- 14.European Commission. Guidance - MDCG Endorsed Documents and Other Guidance. Available at: https://ec.europa.eu/health/md_sector/new_regulations/guidance_en. Accessed March 5, 2022.
- 15.Biomedical Alliance in Europe. Task Force on In Vitro Diagnostics. Available at: https://www.biomedeurope.org/regulatory-affairs/working-group-on-in-vitro-diagnostics.html. Accessed March 5, 2022.
- 16.Bank PCD, Jacobs LHJ, van den Berg SAA, et al. The end of the laboratory developed test as we know it? Recommendations from a national multidisciplinary taskforce of laboratory specialists on the interpretation of the IVDR and its complications. Clin Chem Lab Med. 2021;59:491–497. [DOI] [PubMed] [Google Scholar]
- 17.Spitzenberger F, Patel J, Gebuhr I, et al. Laboratory-developed tests: design of a regulatory strategy in compliance with the International State-of-the-Art and the Regulation (EU) 2017/746 (EU IVDR [In Vitro Diagnostic Medical Device Regulation]). Ther Innov Regul Sci. 2022;56:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Parliament. European Parliament Legislative Resolution of 15 December 2021 on the Proposal for a Regulation of the European Parliament and of the Council Amending Regulation (EU) 2017/746 As Regards Transitional Provisions for Certain In Vitro Diagnostic Medical Devices and Deferred Application of Requirements for In-house Devices. 2021. Available at: https://www.europarl.europa.eu/doceo/document/TA-9-2021-0498_EN.html. Accessed March 5, 2022.
- 19.European Commission. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Amending Regulation (EU) 2017/746 As Regards Transitional Provisions for Certain In Vitro Diagnostic Medical Devices and Deferred Application of Requirements for In-house Devices (COM/2021/627 Final). 2021. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52021PC0627&qid=1634552565285. Accessed March 5, 2022.
- 20.Vogeser M, Brüggemann M, Lennerz J, et al. Laboratory-developed tests in the New European Union 2017/746 Regulation: opportunities and risks. Clin Chem. 2021;68:40–42. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmüller P, Brüggemann M, Eggermann T, et al. Advisory opinion of the AWMF Ad hoc Commission In-vitro Diagnostic Medical Devices regarding in-vitro diagnostic medical devices manufactured and used only within health institutions established in the Union according to Regulation (EU) 2017/746 (IVDR). Ger Med Sci. 2021;19:Doc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (Association of the Scientific Medical Societies). Ad-hoc-Kommission “In Vitro-Diagnostik.” Available at: https://www.awmf.org/die-awmf/kommissionen/nutzenbewertung/ad-hoc-kommission-bewertung-von-medizinprodukten/ad-hoc-kommission-in-vitro-diagnostik.html. Accessed March 5, 2022. [DOI] [PubMed]
- 23.European Scientific foundation for Laboratory Hemato Oncology. IVDR Useful Resources. Available at: https://eslho.org/ivdr/useful-resources. Accessed March 5, 2022.
- 24.European Federation of Clinical Chemistry and Laboratory Medicine. Working Group: Test Evaluation. Available at: https://www.eflm.eu/site/page/a/1158. Accessed March 5, 2022.
- 25.Medical Device Coordination Group. MDCG 2020-5 Clinical Evaluation - Equivalence: A Guide for Manufacturers and Notified Bodies. 2020. Available at: https://ec.europa.eu/health/system/files/2020-09/md_mdcg_2020_5_guidance_clinical_evaluation_equivalence_en_0.pdf. Accessed March 5, 2022.
- 26.EuroBloodNet. EuroBloodNet Homepage. Available at: https://eurobloodnet.eu. Accessed March 5, 2022.
- 27.Biomedical Alliance in Europe. Main Findings IVDR Questionnaire BioMed Alliance. 2021. Available at: https://www.biomedeurope.org/images/news/2021/20211206_Findings_IVDR_Questionnaire_final.pdf. Accessed March 5, 2022.
- 28.European Commission. European Reference Networks. Available at: https://ec.europa.eu/health/european-reference-networks_en. Accessed March 5, 2022.