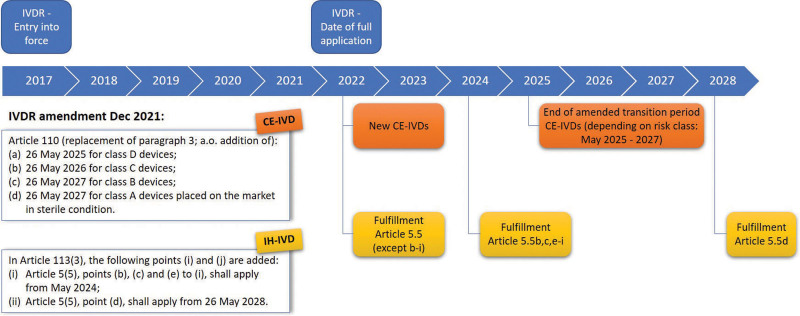
Figure 3.
Timelines for revised phased IVDR implementation. The General Safety and Performance Requirements specified in Annex I as well as Article 5.5 (with the exception of 5.5b to i) are not mentioned in the amendment and are, as such, applicable from May 2022. CE-IVDs = CE marked in vitro diagnostics; IH-IVDs = in-house in vitro diagnostics; IVDR = Regulation (EU) 2017/746 on in vitro diagnostic medical devices.