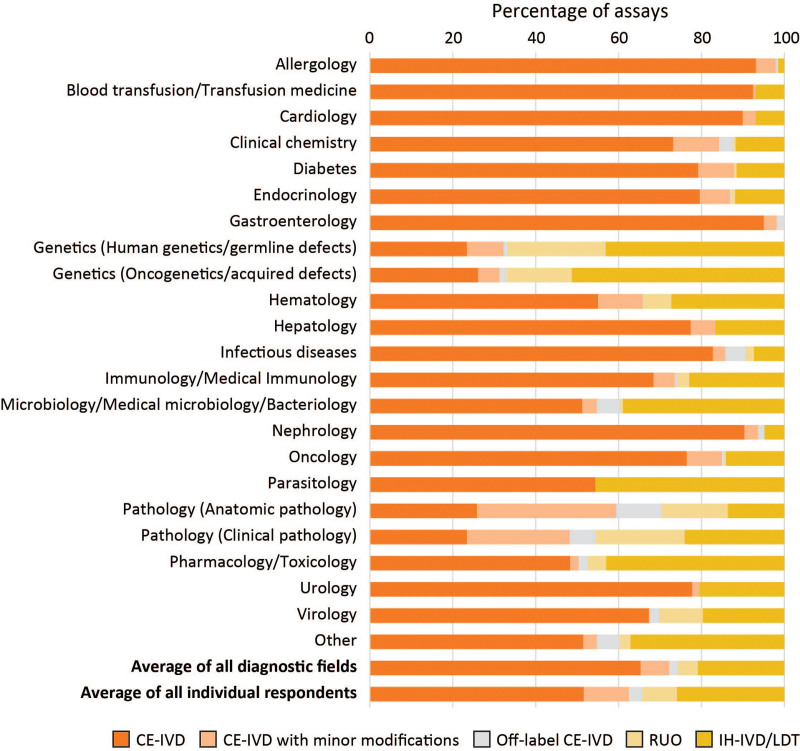
Figure 4.
Average percentage of assays from 5 IVD categories used by respondents of the questionnaire (n = 150), per diagnostic field. (1) CE-IVDs used strictly according to the manufacturer’s IFU; (2) CE-IVDs with minor modifications; (3) Off-label CE-IVDs; (4) RUO kits; and (5) In-house devices (IH-IVDs)/LDTs. A detailed explanation of the 5 IVD categories can be can be found in the main text and the report on the BioMed Alliance website.27 CE-IVDs = CE marked in vitro diagnostics; IFU = instructions for use; IH-IVDs = in-house in vitro diagnostics; IVD = in vitro diagnostic; LDT = laboratory-developed test; RUO = Research Use Only.