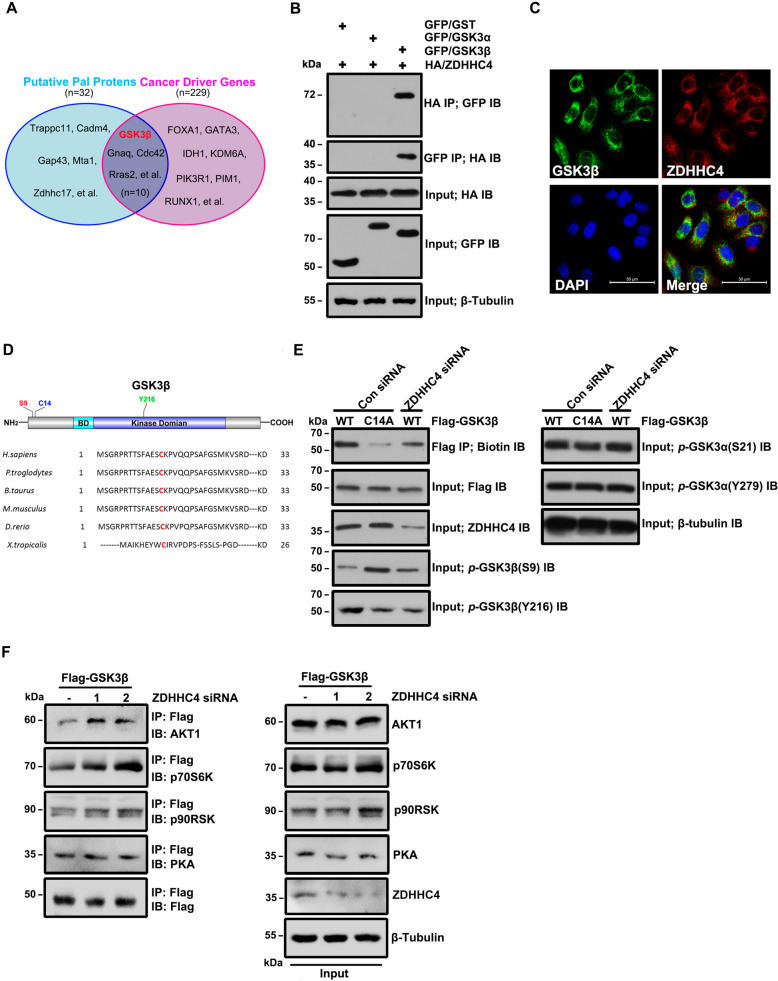
Fig. 1. ZDHHC4-mediated palmitoylation alters GSK3β phosphorylation.
A The collection of palmitoylated proteins and cancer driver genes in GBM. B The interaction of HA-ZDHHC4 with GFP-GSK3α and GFP-GSK3β was verified by immunoprecipitation in 293 T cells. C The localization of ZDHHC4 and GSK3β in SF126 cells was detected by immunofluorescence staining. D Protein map showing the three post-translationally modified amino acid residues in GSK3β. E SF126 cells were transfected, and the experiment was divided into three groups: wild-type Flag-GSK3β, C14A mutant GSK3β, and wild-type Flag-GSK3β were simultaneously knocked down ZDHHC4 by siRNA. ABE analysis and phosphorylation of GSK3α and GSK3β were performed in the three groups. The experiment was repeated twice. F ZDHHC4 was knocked down by siRNA in SF126 cells with stable expression of Flag-GSK3β. Immunoprecipitation assays showed the interaction of Flag-GSK3β with AKT, p70S6K, PKA, and p90RSK.